Malaria is a devastating mosquito-borne disease caused by Plasmodium parasites (Pf). The Pf sporozoite represents a bottleneck in the parasite’s life cycle and is an important target for malaria interventions. The most advanced anti-sporozoite strategies to date, including the World Health Organization-approved RTS,S/AS01 and R21/MM vaccines and monoclonal antibodies (mAbs) developed as clinical products, all target the P. falciparum circumsporozoite protein (PfCSP). Although the sporozoite surface likely has other usable antigenic targets for vaccine and mAb therapeutics, most interventions targeting sporozoites focus only on these well-defined PfCSP repeat regions.
To fill this gap, Joshua Tan’s team at the NIH developed an antigen-agnostic antibody discovery pipeline. This innovative approach used the Beacon® platform to screen antibodies produced by single human B cells that bind to intact Pf sporozoites but not to PfCSP. This method is not limited by a priori knowledge of target epitopes, allowing for the discovery of functional mAbs targeting other epitopes on the sporozoite surface. Their research, published in the January issue of Science (1), identified the cryptic pGlu-CSP site on the surface of Pf sporozoites as a target for protective antibodies.

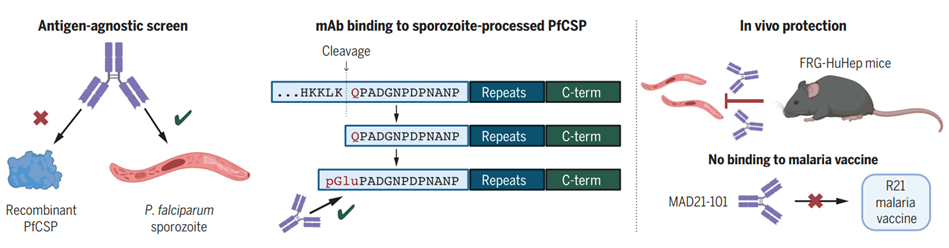
Figure 1. Antibodies targeting the sporozoite pGlu-CSP site can prevent malaria.
Antigen-Agnostic Isolation of Monoclonal Antibodies Targeting Pf Sporozoites
To discover new targets on the surface of intact Pf sporozoites other than PfCSP, researchers screened the plasma of 941 Pf sporozoites-exposed individuals. Their plasma was pre-treated with full-length recombinant PfCSP (rPfCSP) to adsorb antibodies specific to rPfCSP. After blocking with rPfCSP, only five donors out of the 941 had plasma that remained IgG-reactive to sporozoites (Figure 2A,B). B cells were collected from these five individuals for mAb discovery.
In the antibody discovery process, researchers first activated and cultured 475,250 IgG+ memory B cells (MBCs) in 384-well plates (20-100 MBCs per well). The binding capacity of the culture supernatants to Pf sporozoites and rPfCSP was tested (Figure 2C). A small fraction of the supernatants (5.1%, n=55) was reactive to sporozoites but negative for binding to rPfCSP (Figure 2D). Approximately 23,000 B cells from these wells were loaded onto the OptoSelect chip on the Beacon system. Using Opto-Electronic Positioning (OEP®) technology, single B cells were loaded into NanoPen® chambers. By injecting intact Pf sporozoites or rPfCSP-coated beads into the channels above the NanoPens, two rounds of binding assays were performed to screen for antibodies that bind only to intact Pf sporozoites but not to rPfCSP (Figure 2C). Individual B cells with this characteristic were exported for sequencing and production of mAbs in recombinant IgG1 form. Using this method, researchers discovered and recombinantly expressed 10 mAbs that strongly bind to Pf sporozoites but have no binding to rPfCSP (Figure 2E). In addition, they demonstrated that these mAbs were able to significantly inhibit sporozoite invasion and traversal activity compared to other control antibodies, which is known to reduce infection.
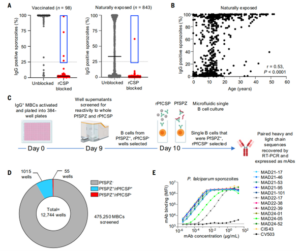
Figure 2. Isolation of rare anti-malarial sporozoite monoclonal antibodies (mAbs) using an antigen-agnostic strategy.
Epitope Identification: Through a series of biochemical and cellular experiments, researchers found that these 10 mAbs bind only to PfCSP expressed by sporozoites, because they require two consecutive parasite-driven modifications of the protein: cleavage of the N-terminus of PfCSP, and conversion of the newly formed N-terminal residue from glutamine to pyroglutamate. Mass spectrometry analysis revealed that pGlu-CSP is a commonly expressed epitope on the sporozoite surface, and naturally occurs in sporozoites, especially in sporozoites derived from salivary glands. In addition, comparison of 16,441 globally isolated Pf parasites from the MalariaGEN Pf7 database for nucleotide polymorphisms, and testing of different variants indicated binding of the MAD24-101 type antibodies were still specific to the most common Ala98Gly mutation.
X-ray Structural Studies: X-ray structural studies of three mAbs revealed that the circulating pyroglutamate residue and adjacent amino acids unique to PfCSP are key binding residues that fit into the pocket formed by pGlu-CSP reactive mAbs. The minimal epitope for these mAbs is pGlu96PADGNP102, which is different from the target epitopes of previously isolated PfCSP reactive mAbs.
mAb Protective Function Analysis: Using the FRG-HuHep human liver chimeric mouse model to assess antibody protective effects, it was found that mAb MAD21-101 provided complete protection against Pf sporozoite infections delivered by mosquito bites. Moreover, MAD21-101 type mAbs do not bind to the malaria vaccine R21 (which uses the same PfCSP sequence as RTS,S), indicating that if used in areas where the vaccine is deployed, these monoclonal antibodies are unlikely to interfere with the function of existing vaccines.
Simultaneous Novel Vaccine Target Epitope and Antibody Therapeutics
This study identified the cryptic pGlu-CSP site on the surface of Pf sporozoites as a target for protective antibodies. According to existing reports, these are the first highly effective anti-sporozoite mAbs that do not bind to the central repeat region of PfCSP. This finding not only deepens our understanding of sporozoite biology but also reveals new targets that can supplement existing interventions. Future malaria vaccines and therapies could potentially incorporate these novel targets to enhance efficacy and broaden immune responses.
“More broadly, this study highlights the benefits of an antigen-agnostic approach to study antibody responses to infectious pathogens, particularly unicellular pathogens with complex proteomes such as P.falciparum. A key advantage of this approach is the potential to identify uncharacterized antigens or epitope features that are not reproduced by recombinant expression, including post-translational modifications… Beyond malaria, the antigen-agnostic strategy deployed in this study could be expanded to other unicellular pathogens with relatively uncharacterized surface antigen profiles, such as Mycobacterium tuberculosis.”
The researchers speculate this antigen-agnostic approach to epitope and antibody discovery can be applied to other pathogenic organisms, including bacteria or even viruses. We remain hopeful researchers can leverage the Beacon platform to combine multiple benefits of the optofluidic single B cell screening, including multiplexable and sequential assays to measure broadly reactive antibodies (2,3), native organism screening, and other biochemical and functional assays, all to accelerate study and preparedness against infectious disease.
References
-
- [1] Dacon C, Moskovitz R, Swearingen K, et al. Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein. Science. 2025;387(6729). doi:https://doi.org/10.1126/science.adr0510
- [2] Fan C, Keeffe JR, Malecek KE, et al. Cross-reactive sarbecovirus antibodies induced by mosaic RBD-nanoparticles. Preprint published online January 3, 2025. doi:https://doi.org/10.1101/2025.01.02.631145
- [3] Dacon C, Tucker C, Peng L, et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science. 2022;377(6607):728-735. doi:https://doi.org/10.1126/science.abq3773