Therapeutic monoclonal antibodies (mAbs) have become less effective against SARS-CoV-2 infections as new variants arise due to point mutations in the spike protein that reduce antibody binding. To induce production of broadly neutralizing antibodies that target a conserved receptor-binding domain (RBD) region, protecting against multiple SARS-CoV-2 variants and zoonotic sarbecoviruses, researchers at the California Institute of Technology developed the Mosaic-8b RBD-nanoparticle vaccine, which displays the RBDs of eight sarbecoviruses on a 60-mer nanoparticle. In the latest pre-print published on bioRxiv (1), the researchers employed the multiplexed antigen-binding assays on the Beacon® optofluidic system to meticulously analyze the antigen reactivity of IgGs induced by the Mosaic-8b nanoparticles in rabbits. They assessed the cross-reactivity of these antibodies and conducted a comprehensive screening to identify antibodies with broad cross-binding capabilities.
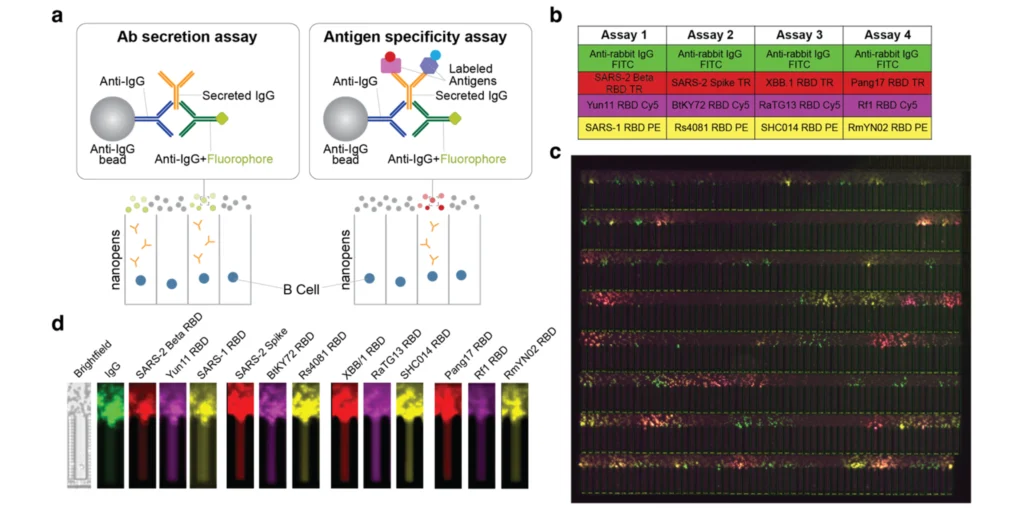
The Beacon Multiplexed Assay Enables Antibody Profiling
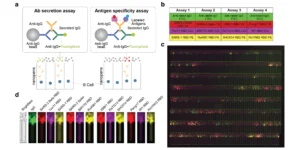
To investigate whether the increased cross-reactivity of mAbs elicited by the Mosaic-8 nanoparticle observed in mice extends to another species, researchers utilized the Beacon optofluidic system to screen approximately 27,000 activated memory B cells from rabbits immunized with Mosaic-8b nanoparticles. The OptoSelect® chips and multiple optical filters on Beacon enabled researchers to conduct multiplexed binding and functional assays of antibody-secreting cells within a single day. In this study, four rounds of multiplex detection were employed, with each round utilizing four different fluorophore-labeled antigens. In all, the system accessed IgG secretion and binding to 12 different antigens for all 27,000 activated memory B cells.
The multiplexed binding assays revealed that rabbits immunized with Mosaic-8b nanoparticles had a higher percentage of B cells recognizing a range of both matched and mismatched receptor-binding domains (RBDs) compared to homotypic SARS-CoV-2 immunization. Specifically, the Mosaic-8b immunization led to a greater proportion of B cells that could recognize multiple RBDs, indicating the production of broadly cross-reactive monoclonal antibodies (mAbs), whereas SARS-CoV-2 immunization predominantly elicited B cells recognizing the matched SARS-CoV-2 Beta RBD. These findings underscore the potential of Mosaic-8b nanoparticles to induce a more versatile immune response, crucial for combating SARS-CoV-2 variants and related sarbecoviruses.
Identification of Antibodies with Broad Cross-Binding Capability
Ultimately, 14 monoclonal antibodies (mAbs) with broad cross-reactivity were exported and amplified, with paired antibody heavy chains and light chains further subcloned into expression vectors. ELISA results showed that three mAbs (M8a-B1, M8a-B9, and M8a-C9) bound to all tested sarbecovirus RBDs with EC50 values <0.1 µg/mL, and several others exhibited broad binding to clade 1a, 1b, and 2 RBDs. Neutralization potencies and breadth of the rabbit mAbs were assessed against a panel of SARS-2 VOCs and other sarbecoviruses, with results generally consistent with binding data. Notably, M8b-A10 (class 4 epitope) and M8b-C9 (class 1/4 epitope) demonstrated both potency and breadth across the tested viruses. Overall, the ELISA results and other functional assays showed strong correlation with the antigen multiplex binding capabilities detected by the Beacon system, highlighting its ability to identify antibodies with broad neutralizing capabilities.
Dual Antibody Therapeutic and Vaccine Development Advances against Infectious Disease
The researchers’ usage of their innovative mosaic-8b RBD nanoparticles demonstrate a highly efficient method to eliciting broadly neutralizing antibodies against multiple known and potentially unknown sarbecoviruses, providing promising paths for both robust antibody therapeutics and novel vaccine target epitopes. By immunizing animals with their unique nanoparticle and leveraging the Beacon system and Bruker Cellular Analysis’ antibody discovery-optimized kits and reagents to rapidly assess cross-reactive binding against 12 different antigens, the researchers were able to efficiently elicit and identify broadly neutralizing antibodies, in contrast to other methods of screening numerous donor samples to identify these rare broadly neutralizing antibodies (2,3,4). In addition, the researchers leveraged these broadly neutralizing antibodies to confirm binding to several conserved epitope classes that were different than the only existing FDA emergency use authorized Pemgarda as of the preprint’s publication. These findings provide potential to support simultaneous development of robust vaccine candidates, vaccine target epitopes, and antibody therapeutics in the continued push to treat and eradicate infectious disease.
Summary
The Mosaic-8b RBD-nanoparticle vaccine effectively elicited broadly neutralizing antibodies in rabbits, and the Beacon platform adeptly identified these antibodies. The multiplexed assay, in conjunction with the high-throughput capabilities of OptoSelect chips, facilitated a detailed characterization of the antibody profiles. This streamlined approach is essential for the development of robust vaccines and therapies against SARS-CoV-2 and its variants.
References
- [1] Fan C, Keeffe JR, Malecek KE, et al. Cross-reactive sarbecovirus antibodies induced by mosaic RBD-nanoparticles. Preprint published online January 3, 2025. doi:https://doi.org/10.1101/2025.01.02.631145
- [2] Dacon C, Tucker C, Peng L, et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science. 2022;377(6607):728-735. doi:https://doi.org/10.1126/science.abq3773
- [3] Hastie KM, Yu X, Ana-Sosa-Batiz F, et al. Potent Omicron-neutralizing antibodies isolated from a patient vaccinated 6 months before Omicron emergence. Cell Reports. 2023;42(5):112421. doi:https://doi.org/10.1016/j.celrep.2023.112421
- [4] Dacon C, Moskovitz R, Swearingen K, et al. Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein. Science. 2025;387(6729). doi:https://doi.org/10.1126/science.adr0510