Amgen on Antibody Discovery and Cell Line Development

— Philip Tagari, Vice President of Therapeutic Discovery, Amgen
As one of the early customers to have adopted the Beacon Platform, Mr. Tagari and his colleagues at Amgen have been able to continuously push the capabilities of the Beacon® optofluidic system. Since adoption, Amgen has expanded their usage of the platform from antibody discovery to cell line development. Mr. Tagari describes the Beacon technology in Amgen’s online feature “We’ve actually put this technology into practice in our antibody discovery work, and it takes about four months off the normal timeline. It’s also our standard method for cell line development, and we’re looking to extend our success in these areas to other applications. It’s starting to feel like a pretty transformative tool.”
>99% Monoclonality Assurance
The Amgen team was also the first to publish a paper on monoclonality of cell lines derived from the Opto® CLD workflows on the Beacon system, sharing with the rest of the scientific community their strategy to validate that clones can be generated with >99% monoclonality assurance, higher overall cloning efficiency than using other well-known methods, offering what they described as a “superior clonality data package”.
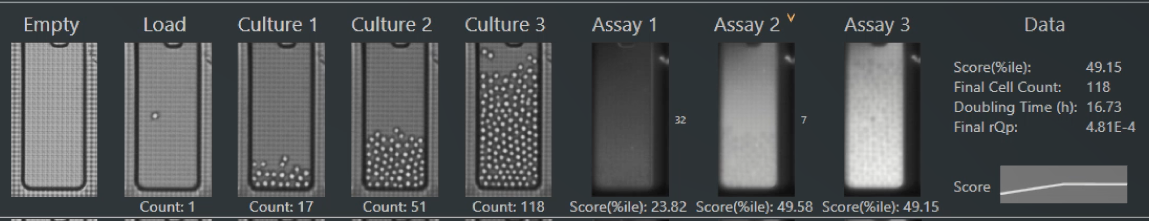
One of the key advantages of the Beacon system is the ability to screen B cells from multiple organs (spleen, bone marrow, lymph nodes) and culture them for multiple days in specialized media to enable multiple screens from a single cell sample.
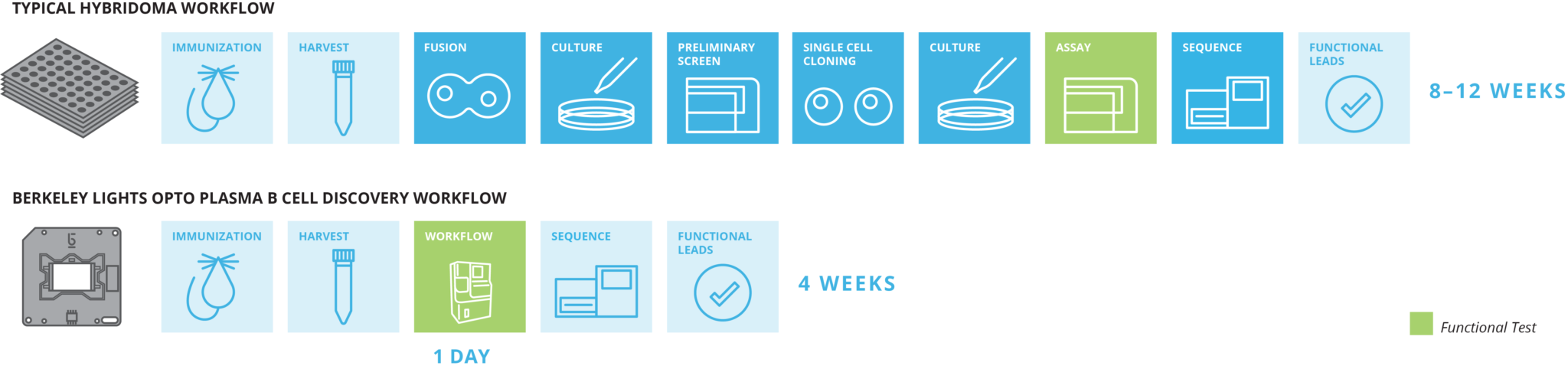
As noted by Amgen’s scientists, using previous methods of hybridoma fusion for antibody discovery starts out with 0.1% of B cells surviving the fusion, not to mention the weeks it takes to grow the millions of cells for screening.
With the Beacon system you can skip the entire hybridoma model, getting to your answers faster and with more relevant data. You can directly assay the B cells and connect antibody function to gene sequence at the single-cell level.