The behavior of early-minted antibody-secreting cells (ASC) or plasmablasts post-infection or vaccination plays a pivotal role in the immune response. Traditionally, long-lived plasma cells (LLPC) from the bone marrow are regarded as non-dividing, while blood ASCs are thought to be proliferative. Traditional technologies that average bulk data or measure surface phenotype alone miss critical functional differences. A new study, published in Scientific Reports by researchers at Emory University, investigates how plasmablast proliferation and antibody secretion map against the traditional model that suggests plasmablasts continue to divide and secrete antibodies. By using Bruker’s optofluidic technology, the study was able to meticulously scrutinize cellular dynamics, challenging established perceptions.
Optofluidic Technology Reveals New Insights
The addition of Bruker’s optofluidic platform in this study allowed researchers to look at both cell division and IgG secretion over longer periods, providing a more complete picture of ASC behavior. Focusing on healthy human ASCs post-vaccination, the study found only about 11% of blood ASCs both divide and secrete IgG antibodies. Surprisingly, these cells stop dividing within 48 hours but continue secreting antibodies daily for 21 days. This differs from bone marrow-derived long-lived plasma cells (LLPCs) that never divide but maintain daily IgG secretion.
The study questions the reliability of Ki67 and BrdU as markers for plasmablast proliferation and IgG secretion. FACS sorting, a common method, falls short by characterizing ASCs based only on surface phenotype, possibly overestimating their numbers.
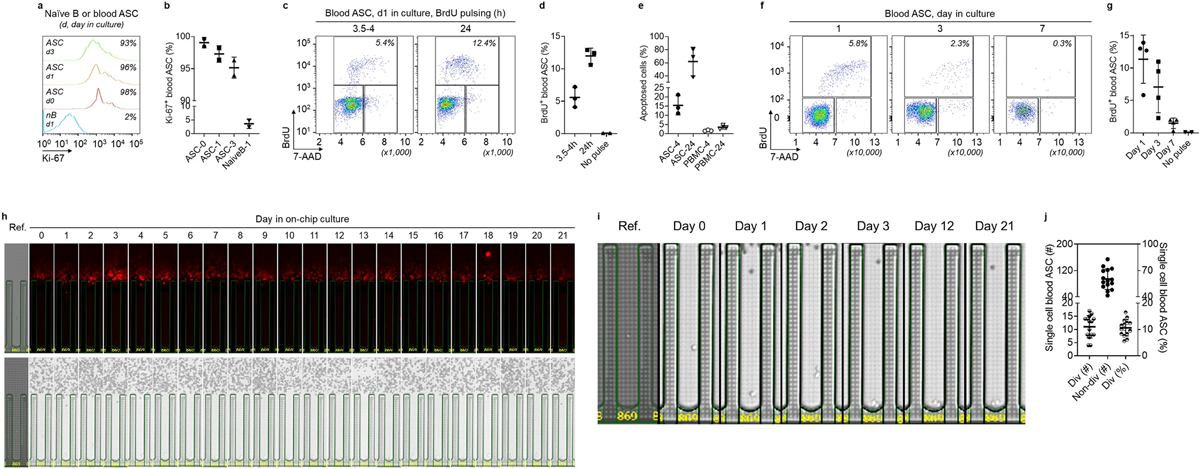
From Nguyen, D.C., Saney, C., Hentenaar, I.T. et al. Majority of human circulating IgG plasmablasts stop blasting in a cell-free pro-survival culture. Sci Rep 14, 3616 (2024). https://doi.org/10.1038/s41598-024-53977-2
Implications and Future Directions:
The findings highlight the need to reevaluate existing ASC classifications. Bruker’s optofluidic platform is a crucial tool, allowing researchers to connect functional characteristics with traditional markers and uncover overlooked signaling and gene expression pathways. This study marks a significant step forward in understanding how these cells work, opening avenues for more precise immunological interventions.
In immunology, embracing cutting-edge technologies like Bruker’s optofluidic platforms is key to unraveling cell complexities. Emory University’s team challenges conventional wisdom, offering a new perspective on antibody-secreting cells. This breakthrough study paves the way for a more precise understanding of ASCs, with potential implications for targeted immunological interventions.