Cancer has long been one of the most challenging diseases to treat as traditional treatments, like chemotherapy and radiation, can be harsh on the body and are not always successful. However, recent advancements in the field of immunotherapy have improved anti-cancer treatments; one of the more promising immunotherapy approaches being Immune Checkpoint Blockade (ICB) therapy.
This cutting-edge approach treats certain types of cancers by blocking checkpoints on immune cells. Crucially, ICBs work by counteracting tumor-associated CD8+ T cell “exhaustion”—a dysfunctional state that arises in response to persistent exposure to tumor cells. When exhausted, CD8+ T cells show a decreased ability to attack cancer cells and an increased expression of cell surface receptors that inhibit the immune response. ICBs can block these inhibitory receptors, allowing CD8+ T cells to remain activated and function properly to destroy tumor cells.
Unfortunately, some cancers like glioblastoma (GBM) do not respond well to ICBs. Cells from these aggressive brain tumors are surrounded by a tumor microenvironment that helps them evade immune cells and by extension, the effects of ICBs. Researchers have observed hypofunctional CD8+ T cells in GBM, but it is unclear whether they are exhausted or in a different hypofunctional state.
To that end, researchers need reliable clinical markers to characterize and distinguish between hypofunctional CD8+ T cell states. Functional single-cell analysis provides key insights needed to design, optimize, and ultimately deploy more effective immunotherapies for GBM and other cancers.
Profiling Hypofunctional States of CD8+ T cells with Single-Cell Analysis
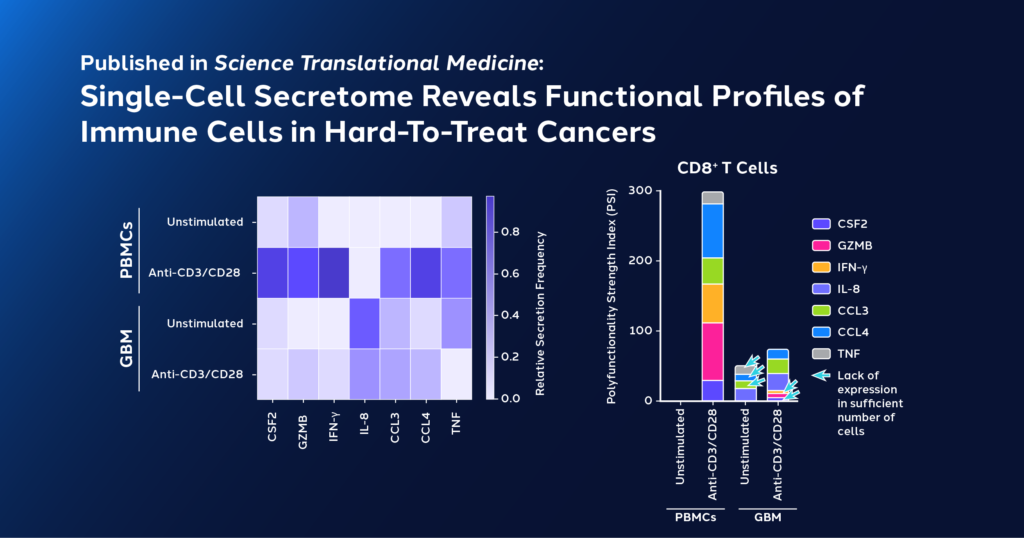
In a recent study published in Science Translational Medicine, researchers used a multiomics and spatial mapping approach to profile hypofunctional states in CD8+ T cells derived from GBM samples and peripheral CD8+ T cells from healthy individuals. CD8+ T cells from both groups were stimulated with anti-CD3/anti-CD28 monoclonal antibodies. Their ability to be activated after exposure to anti-CD3/anti-CD28 was assessed with the IsoCode Single-Cell Secretome Adaptive Immune Platform, which can measure multiplexed cytokine secretions from individual T cells to characterize functional phenotypes and identify key drivers of tumor resistance.
Results showed important differences between the functional phenotypes of healthy control and GBM-CD8+ T cells with and without stimulation:
- Healthy control CD8+ T cells secreted a more functionally diverse set of cytokines and chemokines, and their polyfunctionality strength index (PSI)—a unique metric that encapsulates cytokine secretion potency from polyfunctional cells—jumped 300x after activation.
- In contrast, GBM-CD8+ T cells showed a much lower functional diversity and PSI. Only a 20x increase in PSI after activation was observed.
- Unlike healthy control CD8+ T cells, the jump in PSI after activation in GBM-CD8+ T cells was not driven by the typical secretion of effector or inflammatory cytokines, but by promyeloid chemokines, such as IL-8, CCL3, and CCL4, which are also observed in wound healing.
- The hypofunctional state of stimulated GBM-CD8+ T cells fit a “tolerized” rather than an exhausted phenotype. In this tolerized state, GBM-CD8+ T cells become less responsive to self-antigens that they are chronically exposed to, less effective at performing their functions, and more susceptible to programmed cell death.
Leveraging Single-Cell Analysis to Discover Diverse Phenotypes and Support Immunotherapy Design
Achieved through the high resolution of Bruker Cellular Analysis’ Single-Cell Secretome Platform, this research helps pave the way for single-cell analysis as a tool for learning how to optimize immunotherapy for cancers. In this study, Single-Cell Secretome analysis provided evidence for diverse CD8+ T cell landscapes across human tumors, helping pave the way for future work that pinpoints how tumors evade treatments in different cancers, and how to combat these immune-resistant landscapes to help more patients with hard-to-treat cancers.
Learn more about the power and precision of Single-Cell Secretome for phenotyping of immune cells: Bruker Cellular Analysis’ Isocode Single-Cell Secretome Solutions.