Bispecific Antibodies: An Emerging Therapeutic Paradigm
Bispecific antibodies (bsAbs) are rapidly gaining momentum in the pharmaceutical industry as a revolutionary class of therapeutics. While monoclonal antibodies (mAbs) have dominated the biologics landscape for decades, bsAbs offer unique therapeutic mechanisms by targeting two different antigens simultaneously. This capability enables novel treatment strategies, particularly in cancer immunotherapy, autoimmune disorders, and beyond.
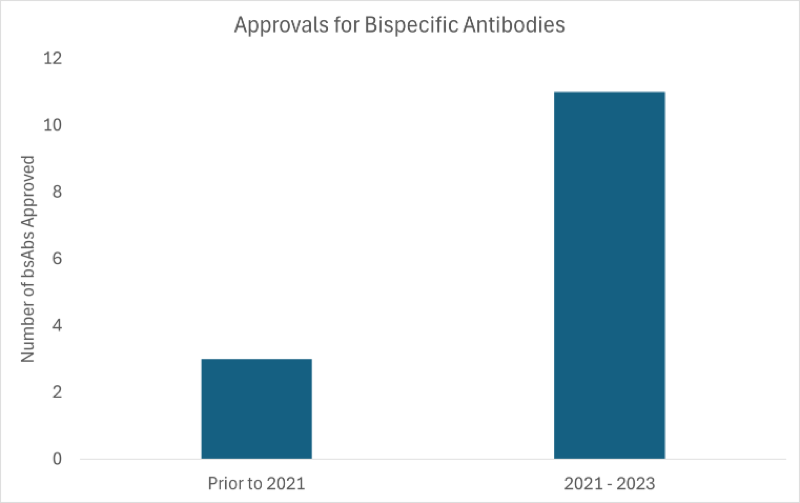
Between 2021 and 2023, the bsAb approval rate surged dramatically. Before 2021, only three bsAbs were approved globally, but from 2021 to 2023, eleven more were added to the growing list of bsAb therapies (Figure 1).
However, developing these complex molecules introduces unique challenges, especially in ensuring quality and productivity during cell line development (CLD). Traditional CLD workflows often neglect to measure critical quality attributes, such as the correct heterodimer concentration, until months post-cloning. Indeed, a recent industry survey found that only 12% of CLD teams assess product quality prior to progressing clones to the agitated culture stage [2]. This delay increases the risk of advancing poor-quality clones, leading to higher costs and wasted resources.
The Beacon® Platform: A Game-Changer for CLD
The Beacon® platform has emerged as a highly advanced, integrated solution for tackling these challenges. Its core technology leverages optoelectronic positioning (OEP) to manipulate particles, allowing precise placement of clones into NanoPen® chambers for real-time analysis.
These NanoPen chambers are central to the platform’s power. Operating at a microscale, each chamber holds less than two nanoliters of fluid. This small volume creates a highly concentrated environment, enabling faster and more accurate detection of secreted antibodies, including bsAbs. For instance, measurements that would take weeks in traditional microwell plates can be achieved within hours to days on the Beacon system.
Additionally, the platform offers precise control over fluidic conditions—such as pH, temperature, and media composition—allowing a closer approximation of bioreactor environments. The unique laminar flow properties within the NanoPen chambers ensure that diffusion, rather than turbulent mixing, drives the transport of molecules, enabling highly controlled and reliable assays [3].
Measuring Bispecifics on the Beacon® Platform
One of the key advantages of the Beacon platform is its ability to screen for bsAbs with high precision. For bispecific molecules that require a specific binding ratio—such as a 1:1 ratio of two distinct binding domains—early identification of clones with the correct heterodimer concentration is crucial. Using the Beacon platform, you can measure the diffusion gradient (DiGr) signal, which correlates directly to antibody production.
Collaborations with industry leaders like GSK and Incyte have demonstrated the effectiveness of this approach. In one study with GSK, 13 different clones were measured using the Beacon platform and compared against high-performance liquid chromatography (HPLC) analysis. The results showed a correlation coefficient of 0.99, confirming the accuracy of the platform’s bispecific measurements.
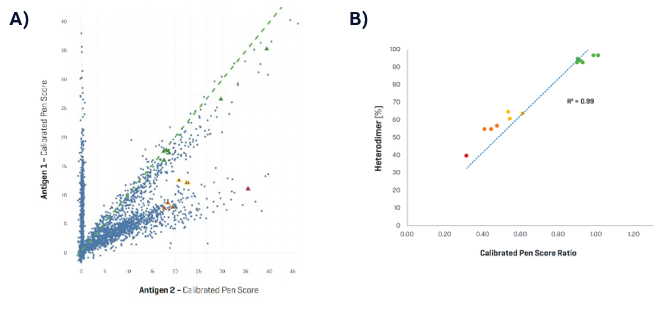
Similarly, in a collaboration with Incyte, the results mirrored these excellent predictive outcomes. For further insights into these capabilities, Dr. Shu Wang from Incyte shared an in-depth analysis during a recent webinar, offering valuable perspectives on bsAb measurements.
SpotLight Reagents: Simplifying bsAb Assays
In 2023, Bruker Cellular Analysis introduced the SpotLight Human Lambda reagent, further streamlining bsAb measurements on the Beacon platform. This addition allows for bsAb assays targeting antibodies with both Kappa and Lambda light chains [4] using off-the-shelf reagents provided by Bruker Cellular Analysis (Figure 3).
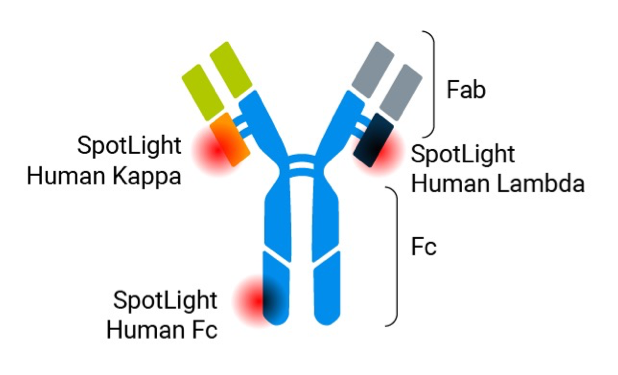
By using SpotLight Human Kappa and SpotLight Human Lambda reagents, users can conduct ratiometric analyses to determine the concentration of each binding domain. This early-stage screening enables the identification of top-producing, high-quality clones within days of single-cell cloning, compared to the weeks or months typically required in conventional workflows.
Conclusion
The Beacon platform is revolutionizing bispecific antibody development. By accelerating the timeline from transfection to top clone identification, researchers can now measure critical quality attributes like heterodimer concentration early in the process. This capability not only reduces costs and development time but also increases the probability of advancing high-quality, productive clones, making the Beacon platform an indispensable tool in the evolving field of bispecific antibody therapeutics.
References
- [1] Surowka, Marlena, et al. “A Pivotal Decade for Bispecific Antibodies?” mAbs, vol. 16, no. 1, 2024, p. 2321635, https://doi.org/10.1080/19420862.2024.2321635.
- [2] Clarke, David C., et al. “When Will We Have a Clone? An Industry Perspective on the Typical CLD Timeline.” Biotechnology Progress, vol. 40, 2024, https://doi.org/10.1002/btpr.3192.
- [3] Sackmann, Eric K., et al. “The Present and Future Role of Microfluidics in Biomedical Research.” Nature, vol. 507, no. 7491, 2014, pp. 181–189, https://doi.org/10.1038/nature13118.
- [4] Fischer, Nicolas, Elson, Greg, et al. “Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG.” Nature Communications, vol. 6, no. 6113, 2015, https://doi.org/10.1038/ncomms7113.