MYCENAX: Developing the Next-Gen Cell Line Development Platform with [Bruker Cellular Analysis]
A leader in CMC development and GMP manufacturing in Taiwan

Mycenax Biotech Inc., established in 2001, is a leading biopharmaceutical company in CMC development and GMP manufacturing in Taiwan. Their expertise includes cell line development, process development, formulation, analytical methods, GMP manufacturing and aseptic filling. With manufacturing facilities based in Taiwan, Mycenax integrates upstream and downstream process development to provide manufacturing service for global customers. Over the years, Mycenax has delivered services for a wide spectrum of different biologics, including, antibody, recombinant protein, vaccine and cell and gene therapy products. Being a leader in process development, Mycenax has always placed emphasis on acquiring innovative state-of-the-art technology platforms to improve service efficiency to their customers. In 2021, they incorporated the Beacon® platform into their cell line development workflow and have since shown great results. Three Taiwan-based and one Japan-based companies have decided to contract with Mycenax for their CDMO projects, and an additional 5–10 companies have shown interests in the Beacon-incorporated CLD platform.
— Molly Shih, Manager of Business Development, Mycenax
Molly Shih, Manager of Business Development, was the group leader responsible for establishing and optimizing Mycenax’s cell line development platform from 2010 to 2019. During this time, she helped dozens of clients generate production cell lines using different hosts and selection systems. In 2019, Molly became a member of the Business Development team where she applies her past experiences in R&D to provide the custom-made project planning for the development of biologics. In this customer spotlight, we share why Molly, together with the Mycenax team, decided to adopt the Beacon® optofluidic system into their cell line development workflow to increase their capabilities and throughput.
Increasing the efficiency of cell line development with evidence-based data
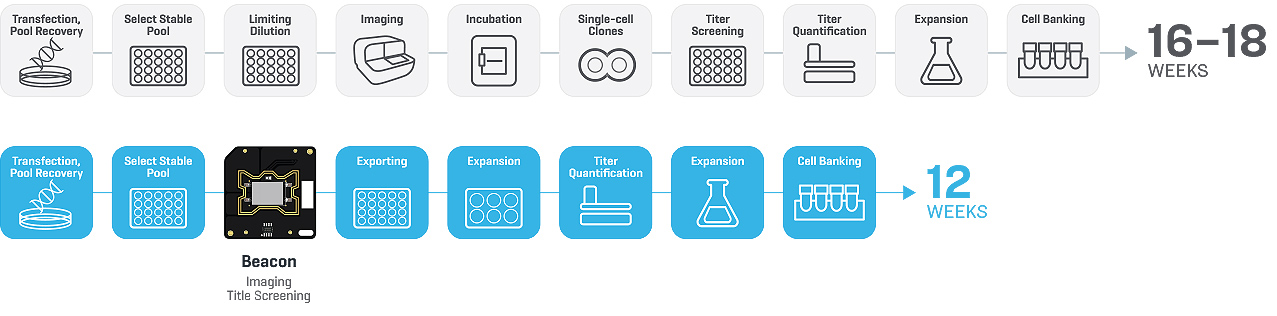
From Molly’s experience, one of the major bottlenecks in cell line development is single-cell cloning. This process requires tedious manual operations to perform limiting dilution, clone imaging, screening and quantitation to select high-yield clones. Traditionally, the overall single-cell cloning workflow took at least 4 weeks to screen approximately a thousand cell clones, using more than 100 96-well plates (FIGURE 1). The Beacon® Optofluidic System offered an attractive solution due to its speed, ease of use and automated workflow.
Molly especially found that the integrated imaging during cell culture on the chip provided direct visual evidence of clonal derivation and saved a significant amount of time and effort. Previously, they had to physically remove each plate one-by-one from the incubator every day to perform imaging to obtain data for monoclonality assurance, resulting in occupational hazards for the operators including visual fatigue and hand tendonitis. “Each time, after hours of looking down the microscope to track every single-cell clone, I always felt exhausted and even started to see cells and wells before falling asleep at night. [Beacon] is a lifesaver!” said Molly. In addition, the data obtained from just a single automated round of cloning on Beacon is known to be more robust compared to their previous method of plate imaging which is sometimes confounded by artifacts and debris, especially along well edges, and contaminating “ghost cells”.
Lastly, the Beacon optofluidic system enabled Mycenax to shorten their cell line development timeline to 3 months and increased their throughput to screen over 4000 cell clones at the same time.

Better CDMO service quality
With this experience in hand, Mycenax started to offer a CLD service incorporating the Beacon optofluidic system for Immunwork, Inc. (http://immunwork.com/, FIGURE 2): Immunwork was founded in 2014, with a unique target-effector platform, which contains “multi-arm linkers” and various “drug bundles” that can be applied to designing and generating drug candidates, including but not limited to antibody-drug conjugates (ADCs), antibody-radionuclide conjugates (ARCs), ultra-long-acting therapeutic peptides and drugs in other modalities. The purpose of this CLD project was to generate a scFv-Fc protein production clone with a research cell bank (RCB) and upstream process for their novel ADC product, TE-1146, targeting multiple myeloma in 8 months.
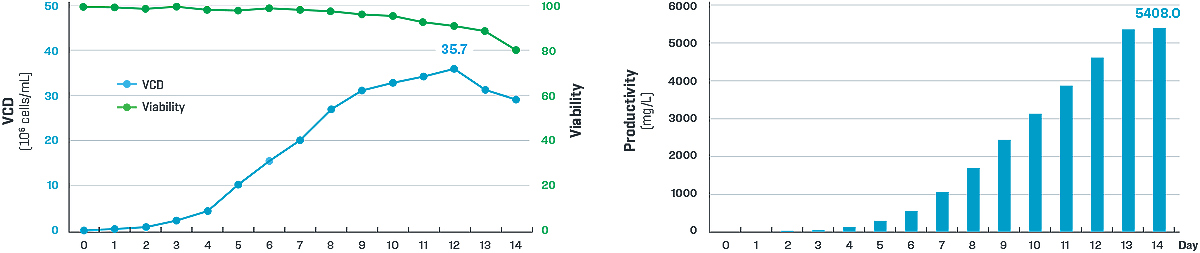
The overall single cell cloning procedure was performed in approximately one week on Beacon, where 57 high-titer (> 2g / L) single-cell clones were selected from over 5000 single cell clones. Among them, 24 cell clones were selected as candidate clones after stability study. After process optimization, one final clone with a titer of 5.4 g/L was obtained (FIGURE 3).
Mycenax was quite satisfied with the result after adapting Beacon in the first CLD cases. It proves that Beacon does save Mycenax in labor and time and helps obtain good-titer-RCB from scratch. With the Beacon optofluidic system, Mycenax is better positioned to provide better CDMO service for CLD in terms of integrated monoclonality data and higher hit rate of high-producing clones.