The Beacon® and Beacon Select™ optofluidic systems accelerate Cell Line Development (CLD), offering rapid cloning, screening, and selection of CHO cell lines.
Widely used by top pharmaceutical firms and CDMOs, these systems enhance workflow efficiency, allowing 4X faster throughput and halving development time while ensuring >99% monoclonality for FDA filings and higher titer clones.
Additionally, Opto™ Assure assays quickly identify quality clones, reducing costs and improving success rates during the scale up process.
Experience Unprecedented Speed
CHO cell line selection is a painful bottleneck in biotherapeutic development, particularly for complex molecules like bispecifics. The Opto® CLD workflow on the Beacon® and Beacon Select™ systems accelerates early CLD by integrating high throughput cell sorting, cloning, culture, productivity, growth, and product quality assays into a single, 5-day automated process. Hear about capabilities of on-chip detection that pinpoints the best clones early in the process.
Cell Line Development (CLD) on the Beacon® and Beacon Select™ optofluidic systems enables high throughput cloning, screening, and selection of top-performing CHO cell lines in just days.
Used globally by leading pharmaceutical companies and CDMOs, the Beacon systems’ industry-leading technology delivers unrivaled speed and efficiency to cell line development workflows. The Opto® CLD workflow has enabled customers to select clones with higher titers than traditional methods, increase throughput by 4X while reducing their cell line development timeline by up to 50%, and recover clones with >99% monoclonality assurance to support FDA IND regulatory filings (Application Note: FDA Accepted IND).
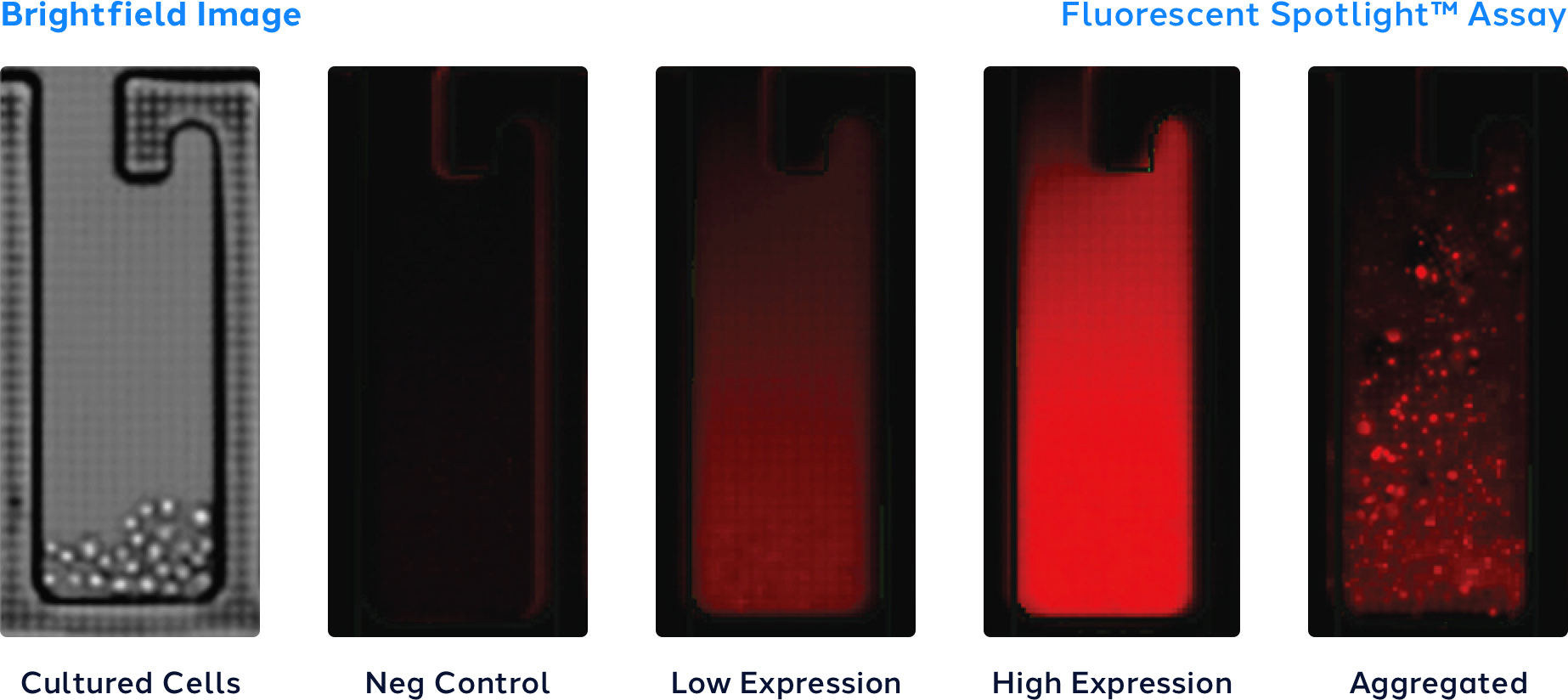
In addition, the Opto™ Assure quality assays enable users to select clones with favorable critical quality attributes within 5 days of cloning to reduce overall costs, improve the probability of success, and further shorten timelines by selecting the top clones for scale up.

Set your IND up for success by getting FDA-accepted monoclonality assurance in just days.

See how pioneers in Cell Line Development use our technology to speed up workflows.
of Cell Line Development
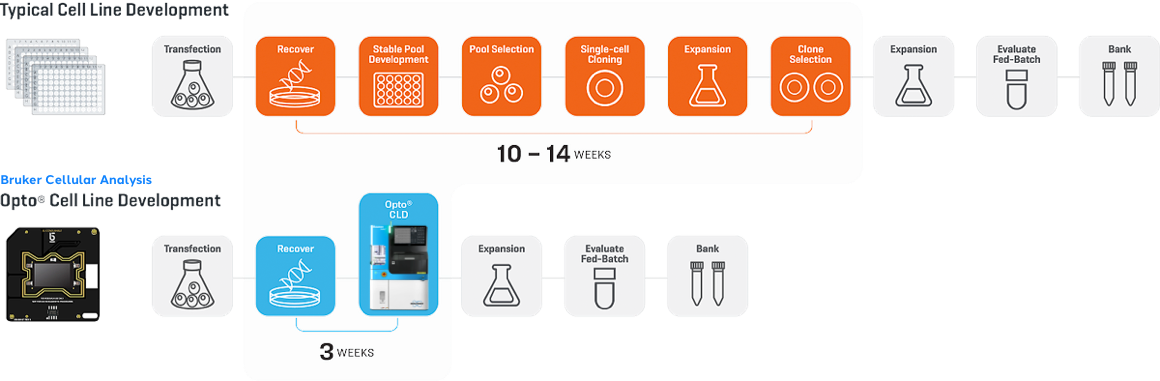
Replace 8-12 weeks of onerous well plate steps with Opto® CLD on the Beacon® and Beacon Select™ optofluidic systems. Screen thousands of clones in parallel and select top clones for even non-traditional antibody molecules, like bispecifics.
Get to production faster with Opto™ Assure for early manufacturability assessment.
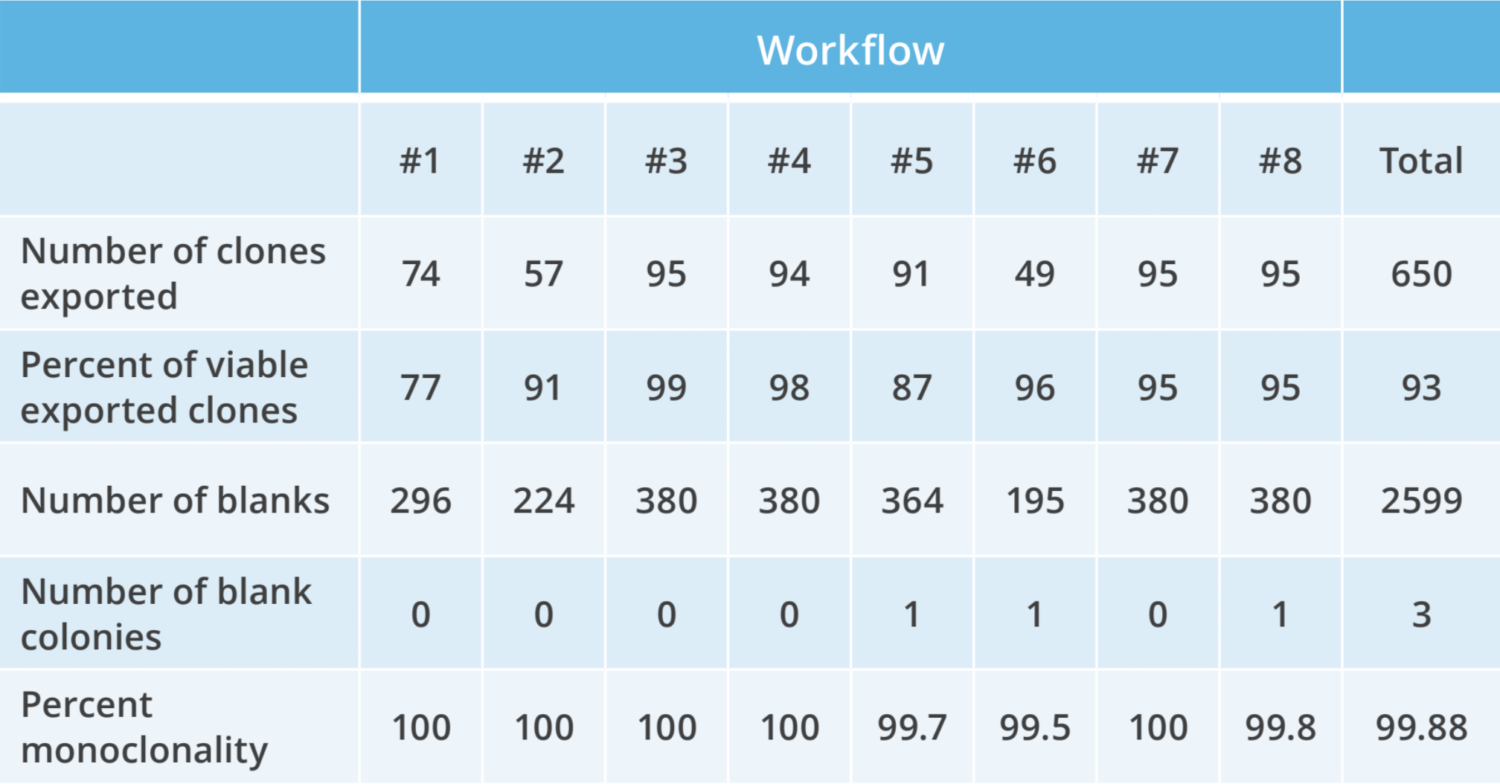
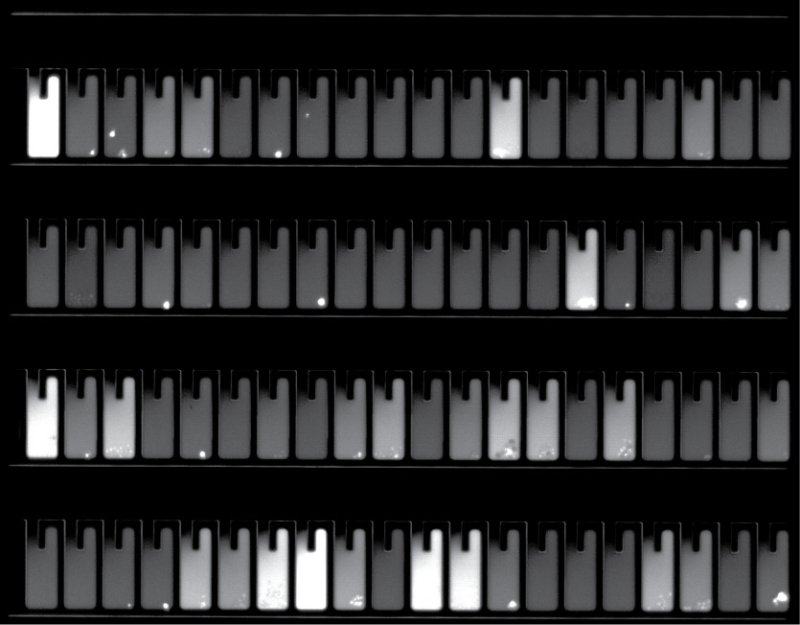
The Bruker Cellular Analysis Opto CLD workflow enables in-line controls to measure clonality during clone recovery. After recovery of each clone, media is flushed into “blank” wells to detect any residual cells. Expand >90% of selected clones from the Beacon system when recovered into 96-well plates, with >99% monoclonality assurance – which is equivalent to four rounds of limiting dilution!
Selective Cell Cloning with Opto CLD lets you screen up to 100,000 cells in a single 5-day workflow, enabling access to more relevant cell diversity than conventional methods for cell line development. Gently enrich transfected pools by identifying and cloning only cells that are viable and express your protein of interest. With Selective Cell Cloning, you can access broad genetic diversity by measuring bulk stable pools, all while eliminating weeks of mini-pool processing.
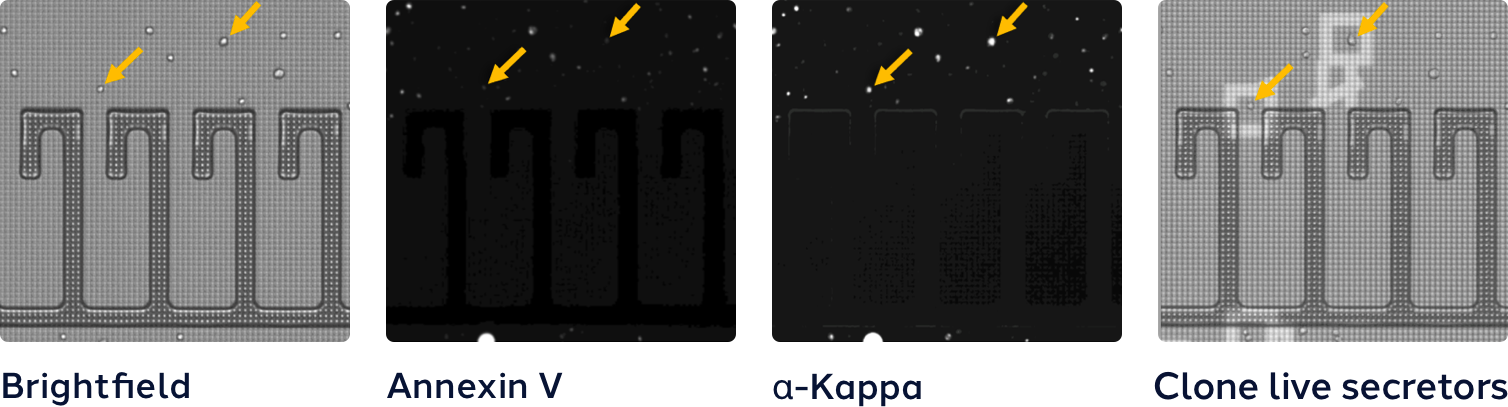
The Beacon system’s optofluidic chip technology and the integrated imaging provide evidence of >99% monoclonality, without manual effort and uncertainty of traditional methods. Learn how the Opto CLD workflow provides superior cloning technology and in-process quality controls for evidence of monoclonality to support regulatory approvals.
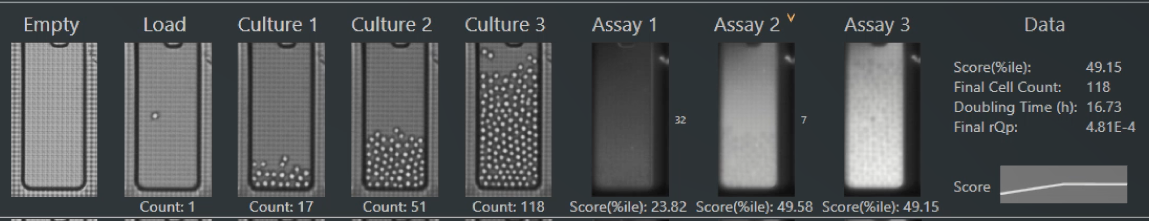
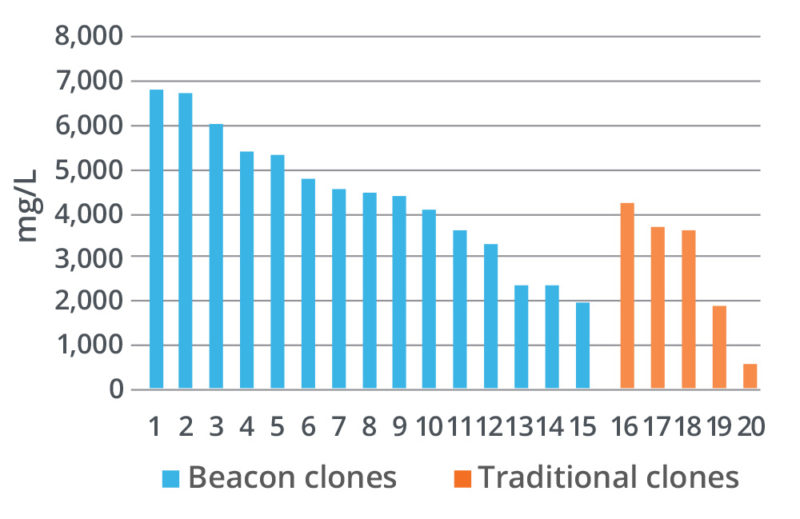
The Opto CLD Workflow enables selection of top clones by measuring growth over multiple days of on-chip culture and secretion titers using quantitative assays for both traditional and non-traditional antibodies.

Resource Type
Instrument
Your CLD Research
Complete the form to connect with us and discover how the Beacon technology can enhance your CLD programs.
Our expert team will be able to answer your questions, show you how the Beacon system works, and share the most relevant datasheets & case studies for your research.