While CAR-T therapies have been revolutionary in the fight against cancer, current FDA approvals for these therapies focus on targeting CD19 or BCMA in B-cell malignancies. Unfortunately, of the patients treated, only up to 50% maintain a sustained response in the long-term. Relapse can occur when cancer adaptively limits CD19 or BCMA expression to render CD19/BCMA-CARs ineffective. Furthermore, suboptimal CAR units and spacing can also affect a CAR’s ability to properly engage target antigen.
Optimizing CAR Constructs to Improve CAR-T Efficacy and Assessing Functionality with Single-Cell Secretome Technology
In a paper recently published in Nature Communications, researchers investigated whether modifying the hinge and transmembrane domains of a novel CAR-T therapy could enhance the efficacy of their product in preclinical models. Upon transduction of the T cells with modified CAR products targeting glypican-1 (GPC1), a surface protein present in many pancreatic cancers, the team found their CAR products to promote effective tumor-killing and overall persistence as shown by the expansion of the CARs in the spleens and pancreas of their preclinical mice.
Though successful in GPC1-presenting cells, the researchers found that the CAR products did not perform at the same level for non-GPC1 presenting tumor cells, and thus wanted to explore how modifying the hinge and transmembrane domains of the CAR would affect the efficacy of the product. The group divided the CARs into three groups of short, medium and large to best assess which length would be most successful, finding that the shorter length (D4-IgG4H-CAR) with a CD28 transmembrane domain produced the greatest cytotoxic potential.
To further test the functional efficacy of the CAR product, the researchers looked to evaluate the polyfunctionality and polyfunctional strength index (the number of cells in their sample secreting two or more types of cytokines multiplied by the signal intensity) to compare CD4 and CD8 CAR products with various hinges and transmembrane domains. Using Bruker Cellular Analysis’ IsoCode Secretome platform, the researchers found that the D4-IgG4H-CD28TM construct had the highest polyfunctional cytokine secretions.
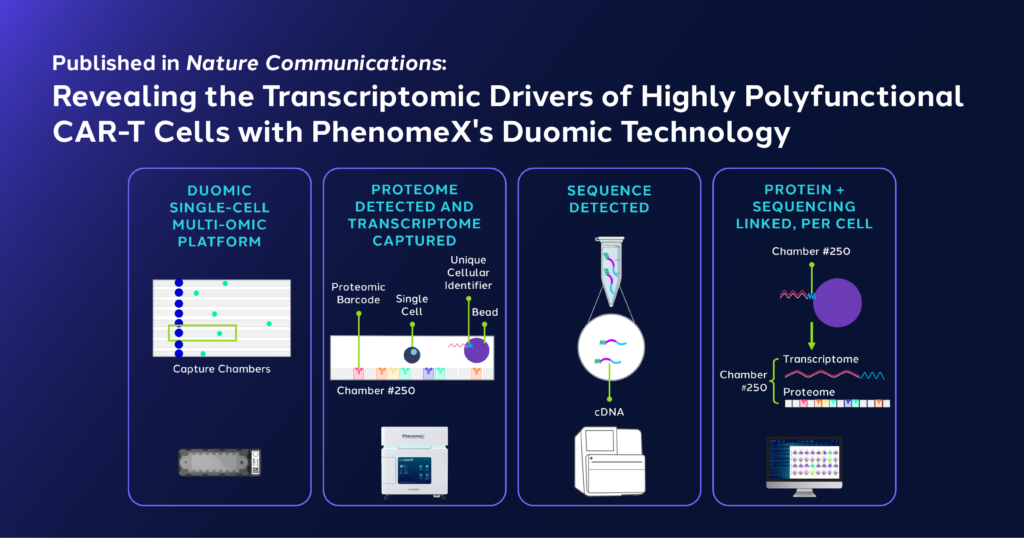
Uncovering the Transcriptomic Drivers of Polyfunctionality at a Single-Cell Level with Duomic
To better understand what was causing increased D4-IgG4H-CD28TM polyfunctionality, the team used Bruker Cellular Analysis’ Duomic chip to simultaneously measure the protein and RNA expression levels of 23 single CD8+ CAR-T cells stimulated with T3M4. Using the premiere platform, the researchers identified two CAR-T clusters, one with high polyfunctionality, with upregulation of the cytokines associated with T cell functionality, and one with low polyfunctionality, with low cytokine levels. They also found 23 genes that displayed statistically significant differences between the two subsets of high and low polyfunctionality, the higher cohort displaying an upregulation of genes involved in promoting cell proliferation, cytokine signaling, and immune responses, all of which are potentially associated with CAR-T cell efficacy.
Using Bruker Cellular Analysis technology to Simultaneously Profile Single-Cell Proteomics and Transcriptomics
With Bruker Cellular Analysis’ breakthrough technology, researchers can identify cells with higher cytotoxicity and effectiveness in treating cancer cells. By using Bruker Cellular Analysis’ Duomic and IsoCode technology, the study showed that the novel CAR-T product was highly efficacious in tumor killing in cell culture and murine models, potentially expediting the development of the next generation of highly efficacious cell therapies.