Monoclonal antibodies are a powerful weapon in the fight against pathogens. However, infectious diseases can quickly develop variants with increased transmissibility or deadliness. In order to keep up with these emerging variants, scientists must be able to quickly identify effective antibodies.
The COVID-19 pandemic has been a prime example of this challenge. The SARS-CoV-2 virus has undergone several mutations, leading to the emergence and global spread of new variants. While some were more lethal and transmissible than others, all were a cause for concern. Unfortunately, many of the antibodies developed early in the pandemic have lost most if not all of their efficacy against these new strains, particular those of the Omicron variety.
Variants of concern (VOCs) have mutations in the spike protein that can enhance the virus’ transmissibility and impact the efficacy of vaccines. The Omicron variant, Omicron BA.1, which surfaced in South Africa in 2021, harbored over 30 mutations in the spike protein, posing a significant threat to vaccine efficacy. As we now know, the epitope landscape of this Omicron variant presented a significant challenge to antibody treatments. Even highly effective FDA-authorized antibodies showed reduced efficacy against one or more Omicron lineages, revealing the magnitude of this threat.
The Bruker Cellular Analysis Beacon® Optofluidic System Enables Early Antibody Discovery
A recent study published in Cell Reports has revealed a panel of vaccine-derived antibodies that possess the ability to fight off various threats. The findings highlight the power and limitations of SARS-CoV-2 antibodies, providing critical guidance for the advancement of broad-spectrum therapeutic treatments.
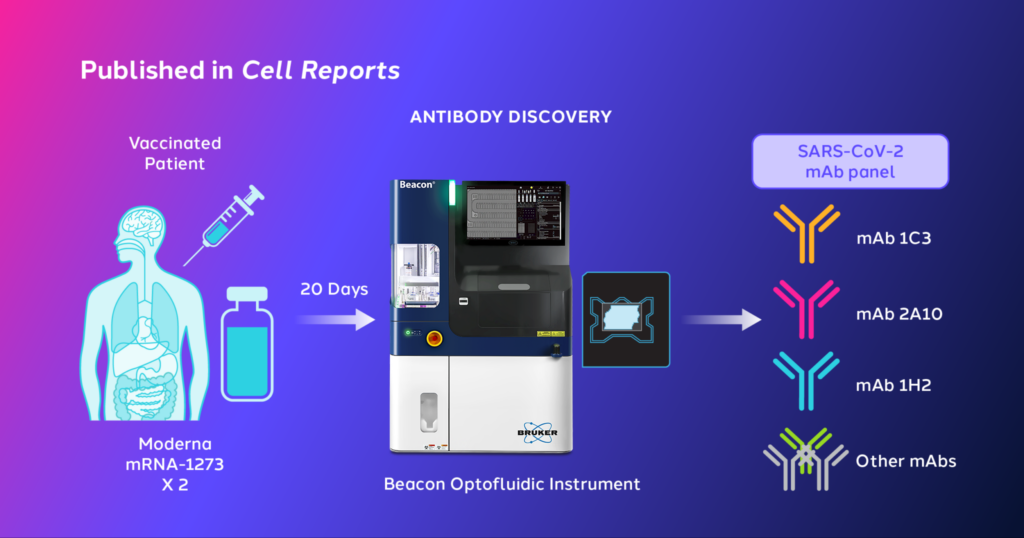
In the study, researchers used the Bruker Cellular Analysis Beacon® Optofluidic System and Antibody Discovery workflow to isolate individual B cells into NanoPen® chamber(s) and screen secreted antibodies for reactivity to Delta-Spike antigens.
Our Beacon system successfully identified antibodies from a vaccinated patient that exhibited broad activity against Omicron VOC six months prior to its emergence. These monoclonal antibodies have been found to be effective in protecting and treating mice infected with BA.1 and BA.2, implying their potential as a treatment for escaped COVID-19 variants. The study outcomes also indicated that vaccination can produce antibodies that are effective against emerging variants of SARS-CoV-2. At the core of the Beacon system is an innovative combination of optics and fluidics called optofluidics. Within the Beacon system, OptoSelect chips replace conventional well plates. Each OptoSelect chip contains thousands of nanoliter-scale chambers, called NanoPen chambers, that enable researchers to characterize cells and identify the best antibody lead candidates.
Single B cells are positioned into individual NanoPen chambers and assays are performed across all NanoPen chambers simultaneously to find the best antibodies in minutes. With tens of thousands of NanoPen chambers, the Beacon system and the OptoSelect chip automate traditionally tedious workflows and increase the odds of finding rare antibodies, reducing both time and cost.
Paving the Way for Next-Generation Vaccines
This study underscores the importance of discovering monoclonal antibodies against new variants in developing effective COVID-19 treatments. By gaining deeper insights into antibody epitopes and patterns, we can pave the way for the next generation of vaccines.
Rapid antibody discovery is critical in accelerating vaccine development and therapeutic interventions aimed at preventing or treating infectious disease. Scientists must have access to innovative tools, like Bruker Cellular Analysis’ Beacon system, to rapidly identify mAbs that can aid in furthering innovative vaccines and therapeutics. With this technology, we can continue to advance towards a safer future.