In a groundbreaking study, researchers at the University of Pennsylvania have identified a novel approach for advancing the effectiveness of CAR-T cell therapy for treating multiple myeloma (MM), a challenging form of cancer. In particular, the study investigates the phenomenon of CAR-T cell suppression, which often leads to relapses in MM patients treated with BCMA-specific CAR-T cells. In a new study published in the journal Blood, researchers including authors Drs. Yan Tang, Wei Liu, Carl June, Neil Sheppard and others, used Bruker Cellular Analysis’ IsoSparkTM Platform to characterize CAR-T cell functionality using the IsoCode® Single Cell Secretome and CodePlex® Bulk Secretome chips.
Understanding the Role of CD200 in MM
The introduction of BCMA-specific CAR-T cell therapies has brought significant changes to the treatment landscape for multiple myeloma (MM). However, a challenge arises as patients who relapse often still have BCMA+ disease, hinting at CAR-T cell exhaustion as a limiting factor. Within the tumor microenvironment of MM, immune checkpoints like CD200 are expressed, contributing to CAR-T cell exhaustion. CD200 interacts with its receptor, CD200R, leading to the suppression of CD200R+ cells, including T and NK cells. This phenomenon is not unique to MM but is observed in various malignancies, both solid and hematological, where the CD200-CD200R pathway is used by tumors to dampen antitumoral immune responses.
Overcoming CD200-Mediated Suppression
The researchers explored the role of CD200 in inhibiting CAR-T cell activity in multiple myeloma (MM). They found that CD200 was prevalent on aberrant plasma cells (aPCs) in MM patients but not on healthy plasma cells. Interestingly, commonly used MM cell lines lacked CD200 expression. By manipulating CD200 levels in MM cell lines, they demonstrated that even low expression levels of CD200 could significantly suppress the cytotoxicity of CAR-T cells targeting TnMUC1, while BCMA CAR-T cells were moderately affected. These findings suggest that CD200 may serve as a critical checkpoint suppressing CAR-T cells in MM, shedding light on potential strategies to enhance CAR-T therapy effectiveness in this context.
Harnessing the CD200R-CD28 Switch for enhanced CAR-T Function
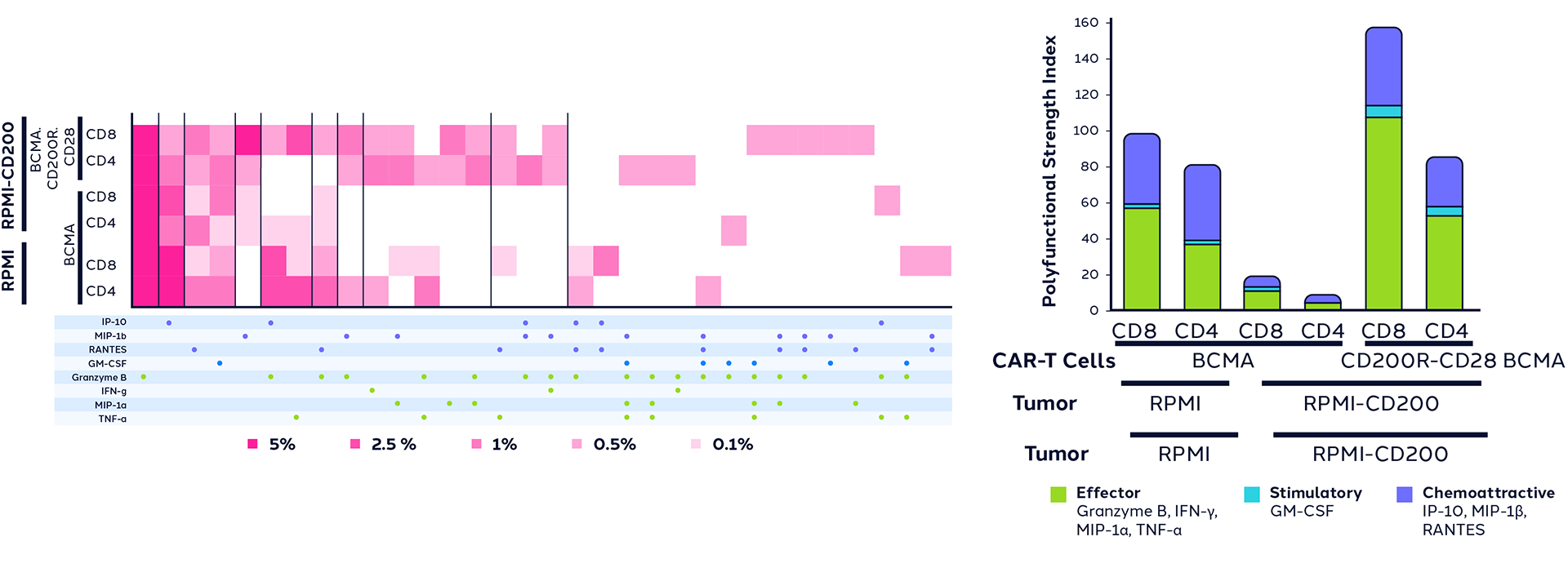
To test their hypotheses, they first engineered MM cell lines to express CD200, an immune checkpoint highly expressed on abnormal plasma cells in MM. This allowed them to explore strategies to overcome CD200-mediated suppression of CAR-T cells.
The study compared different approaches, including CRISPR-Cas9 knockout of the CD200 receptor (CD200RKO), using non-signaling versions of the CD200 receptor (CD200RDN), and a CD200R-CD28 switch connecting CD200R activation to CD28 co-stimulatory signaling. Using Bruker Cellular Analysis’ IsoSparkTM Platform, researchers characterized the CAR-T cell polyfunctionality as well as cell supernatants using IsoCode® Single Cell Secretome and CodePlex® Bulk Secretome chips, respectively. Results revealed that the CD200R-CD28 switch significantly improved CAR-T cell polyfunctionality, enhancing cytotoxicity, proliferation, metabolism, and performance in chronic antigen exposure tests.

The Promise of CD200R-CD28 Switch-Equipped CAR-T Cells
The CD200R-CD28 switch-equipped CAR-T cells exhibited remarkable enhancements in their functionality. They displayed increased cytotoxicity against CD200+ MM cells and superior survival rates in murine xenograft models of MM. Moreover, these augmented CAR-T cells showed a heightened ability to accumulate in the bloodstream, providing a potential game-changer in MM treatment.
Implications and Future Prospects
These findings highlight the importance of CD200-mediated immune suppression in MM CAR-T therapy and offer a promising approach to enhance CAR-T therapies by utilizing the CD200R-CD28 switch to provide co-stimulation via CD200 expression on abnormal plasma cells.
The study results open up exciting possibilities for the optimization of CAR-T therapy in MM, shedding light on the crucial role of the CD200-CD200R checkpoint pathway in resistance to CAR-T therapy. The CD200R-CD28 switch, which can be activated at relevant levels of CD200 on cancerous cells, offers a promising avenue for clinical translation. By enhancing CAR-T cell metabolism, proliferation, and cytokine production, this innovative approach could pave the way for more effective MM treatment strategies. Furthermore, the study suggests that the CD200R ectodomain might have an unappreciated role in T cell metabolism, unveiling new facets of immune system dynamics. Overall, this research provides a strong rationale for further exploration of the CD200R-CD28 switch in the clinic, not only for MM but also for other indications, offering renewed hope for patients battling this challenging disease.