In the rapidly evolving landscape of cancer treatment, Chimeric Antigen Receptor T cell (CAR-T) therapy has emerged as a promising strategy for patients with certain hematologic malignancies. However, the occurrence of relapse has proved challenging. In a recent publication in the Journal for ImmunoTherapy of Cancer, researchers from University College London investigated the potential capability of AKT Inhibitor VIII (VIII) to enhance CAR-T cell fitness. The researchers aimed to determine whether incorporating VIII into the CAR-T manufacturing process could effectively mitigate the risk of relapse and enhance overall patient outcomes.
Enhanced Polyfunctionality Revealed
The study examined whether incorporating VIII into the manufacturing process could improve the presence of functional stem-cell memory and central memory T cells by separating T cell expansion from differentiation, potentially reducing the risk of relapse.
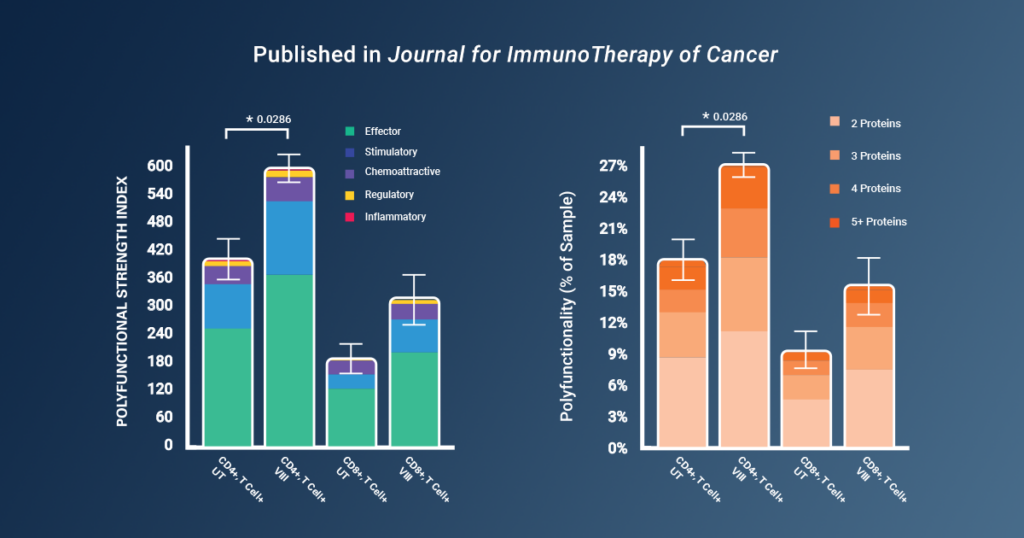
Using Bruker’s IsoCode® Single-Cell Secretome technology, the researchers were able to analyze the cytokine secretions from single CAR-T cells and compare CAR-T cells manufactured with and without VIII. The results of the study revealed a significant enhancement in CAR-T cell polyfunctionality when VIII was incorporated into the manufacturing process. Single-cell analysis of cytokine secretions demonstrated a 1.6-fold increase in Polyfunctional Strength Index (PSI) in CAR-T cells manufactured with VIII. Importantly, the polyfunctional secretion of effector and stimulatory cytokines from both CD4+ and CD8+ CAR-Ts was heightened, without a corresponding increase in regulatory cytokine secretion.
The Role of Proteomic Barcoding in Assessing Fitness
By using Bruker’s proteomic barcoding platform to gather valuable data on polyfunctionality, the study was able to uncover key insights into the fitness of CAR-T products.
“Of note, discrimination of the functional benefits of VIII was not obvious here in vitro by conventional cytotoxicity assay, but instead by our novel rechallenge stress test and the readouts from the [Bruker] polyfunctionality platform.” *
Unlocking Therapeutic Potential
This study sheds light on the potential of VIII to improve CAR-T cell fitness and reduce the risk of relapse in patients. The integration of VIII into the manufacturing process demonstrated enhanced polyfunctionality, particularly in effector and stimulatory cytokine secretion, without a concomitant increase in regulatory cytokines. The findings underscore the importance of innovative approaches, such as the IsoCode® Single-Cell Secretome technology, in advancing our understanding of CAR-T therapy and moving closer to achieving improved progression-free survival rates for patients.
*, et al AKT inhibition generates potent polyfunctional clinical grade AUTO1 CAR T-cells, enhancing function and survival