In cancer immunotherapy, TCR-T based cell therapies show promise for addressing specific types of solid tumors; however, variable patient responses and the potential for relapse indicate a need for improvement. A recent study published in Cancer Immunology Research by researchers at UCLA investigates clinical biomarkers for patient response in TCR-T therapy, shedding light on crucial insights that could influence the success of these therapies.
Investigating the Proteomic Puzzle
The study focused on understanding the key factors influencing the success of TCR-T therapy by examining single-cell cytokine secretions, single-cell phosphoproteins, and bulk cytokines. To unravel this complex proteomic puzzle, the researchers employed Bruker’s IsoCode® Single-Cell Secretome, IsoCode® Single-Cell Signaling, and CodePlex® technology, respectively.
The research specifically evaluated cells generated for patients receiving MART-1 and NY-ESO-1 TCR-T cells in clinical trials. Leveraging the Bruker’s Proteomic Barcoding platform, the study compared functional secretion, phosphoproteins, and bulk cytokines of TCR-T therapy recipients, offering a panoramic view of the proteomic landscape associated with clinical response.
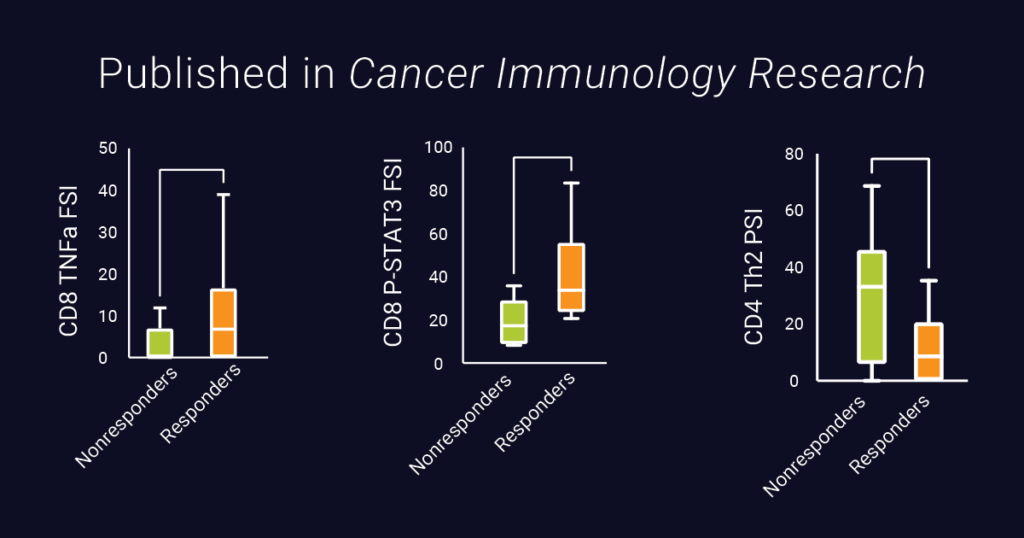
The results revealed correlations between specific proteomic activities and clinical outcomes. Notably, functional secretion of TNF-a from CD8+ T cells and increased STAT3 activity were significantly associated with superior clinical response and patient survival. This discovery suggests a potential avenue for enhancing TCR-T designs in the future.
Additionally, the study revealed that greater CD4+ Th2 polyfunctional cytokine activity is associated with an inferior clinical response and an increased percent change in tumor burden, suggesting that Th2 activity could potentially interfere with TCR-T therapy.
Empowering Future TCR-T Designs
The researchers emphasize the significance of their proteomic approach, stating that, “the single-cell characterization of T-cell cytokine polyfunctionality and signaling activity employed by our study, which we believe to be the first of its kind in clinical TCR T-cell therapy products, enables greater resolution of specific cell populations and their cytokine producing/signaling activities that might be lost in traditional bulk assays. By interrogating the cytokine functionality and signaling elements associated with clinical response, and degree of clinical responsiveness to therapy, our exploratory, discovery-based studies [have] identified numerous potential targets for enrichment or exclusion in future generations of TCR T-cell therapies for solid malignancies.”
The exploration into TCR-T therapy using multiplexed proteomic analysis has illuminated critical pathways and biomarkers that influence clinical response. The study’s findings not only deepen our understanding of the mechanisms behind TCR-T therapy success but also provide a roadmap for refining and advancing future generations of TCR-T cell therapies for solid tumors. As the field of cancer immunology progresses, these discoveries hold the promise of transforming TCR-T therapy into a more precise and effective tool.