The use of therapeutic cancer vaccines is a promising immunotherapy option designed to activate immune effectors against tumor antigens. Research into the use of mRNA as a cancer vaccine platform spans back to the 1990s. Unfortunately, the short half-life of mRNA results in transient protein expression that limits its effectiveness as a cancer vaccine. This guided researchers to look to a different type of RNA, known as self-replicating, single-stranded, positive sense RNA (srRNA). In comparison to mRNA, srRNA generates more highly persistent cancer vaccines with larger amounts of antigen generation maintained over a longer period of time, making it a strong option for use in future cancer vaccine development.
As researchers look into more application for srRNA, identifying how cancer vaccines perform throughout the various stages of development is increasingly important, particularly with regards to safety and the ability to generate robust cancer specific immune responses. Bruker Cellular Analysis’ IsoCode® Single-Cell Secretome and CodePlex Secretome platforms help researchers fully analyze the functional profile of each individual cell and entire bulk populations, providing deeper insights into therapeutic efficacy and the mechanisms driving anti-tumor immune response.
Single-Cell and Bulk Functional Proteomics Reveal Key Response following Combination Therapy
In a recent review article published in Cancer Gene Therapy, the authors discuss previous clinical trials involving srRNA-based cancer vaccines and introduced their own study investigating srRNA-based cancer vaccines targeting the HER2 biomarker in breast cancer patients in conjunction with pembrolizumab, an anti-PD-1 checkpoint inhibitor. Based on previous research which showed an increase in anti-tumor activity for the HER2 cancer vaccine when combined with anti-PD-1 antibodies, the researchers looked to compare differences in plasma and cellular immune profiles before versus after treatment.
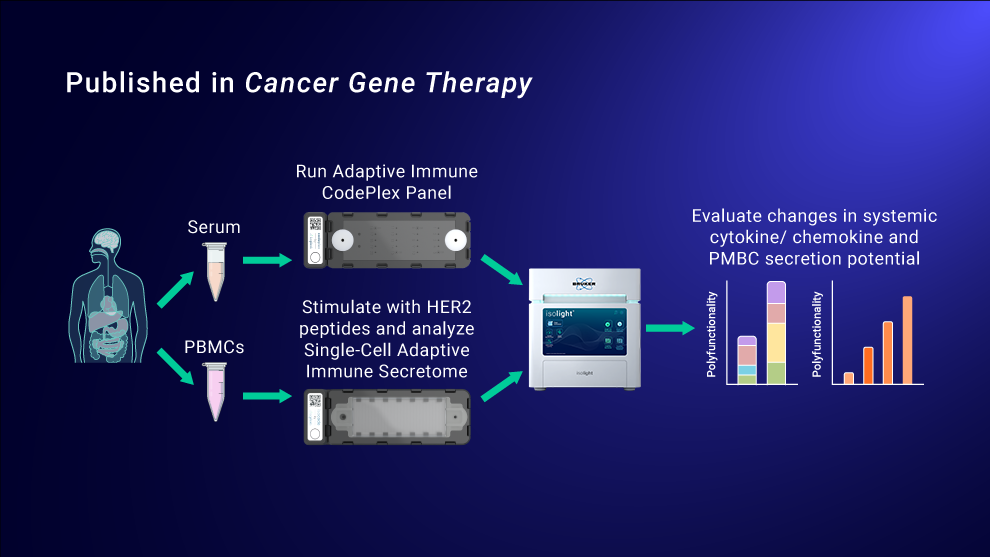
To better understand and characterize the effects of the combined treatment, the researchers collected pre- versus post-treatment serum from patients and analyzed the samples using Bruker Cellular Analysis’ CodePlex Secretome Platform in order to detect bulk cytokine levels. Analysis revealed greatly improved circulating levels of chemokines and CD8 activation molecules MIP-1b, IP-10 and Perforin, in patients treated with both the HER2 cancer vaccine and pembrolizumab, indicating an increased immune response following combined treatment.
The researchers also wanted to investigate pre- and post- treatment response at the single-cell level. Using Bruker Cellular Analysis’ IsoCode® Single-Cell Secretome platform, researchers analyzed PBMCs from each patient and found that the combined treatment increased the polyfunctional strength index (PSI), the percentage of polyfunctional cells multiplied by average signal intensity, and specifically augmented polyfunctional secretion of effector, stimulatory, and regulatory cytokines. These preliminary results established that HER2 cancer vaccine administration with pembrolizumab treatment successfully enhances the polyfunctional capabilities of single cells which has correlated with treatment efficacy in multiple cancer vaccine and immunotherapy studies.
Using Functional Proteomics to Investigate Therapeutic Efficacy
This study demonstrates how using both single-cell and bulk functional analysis can lead to insights into the efficacy of different forms of anti-cancer treatments, helping to determine the effectiveness of each treatment against advanced cancer types. Understanding the functional mechanisms of srRNA-based cancer vaccines combined with an anti-PD-1 checkpoint inhibitor has the potential to empower researchers to develop stronger, more effective therapies that provide increased response and clinical success. Single-cell and bulk functional analyses are being used across a wide variety of applications and diseases to advance human health and develop better therapies.