High-throughput screening (HTS) and DNA-encoded library (DEL) screening have each transformed early small molecule drug discovery, but historically, they’ve operated in parallel rather than in concert. Traditional DEL screening excels at identifying binders, while phenotypic HTS is relied upon to determine function. Bridging those two worlds with confidence and at scale has remained a persistent challenge.
A recent bioRxiv preprint introduces a compelling new approach that begins to close this gap. In “PhenoDEL as a Novel Screening Strategy Based on Intracellular Protein Degradation Activity,” the authors demonstrate a proof-of-concept workflow that combines one-bead one-compound (OBOC) DNA-encoded libraries with the Beacon® optofluidic platform to enable function-first, phenotypic small molecule screening at single-cell resolution [1].
The result is a glimpse of how optofluidics can fundamentally expand the role of DEL and redefine how functional small molecule screening is performed.
What Limits Traditional DNA‑Encoded Library (DEL) Screening?
DEL technology has become a mainstay of modern medicinal chemistry, prized for its ability to interrogate millions to billions of compounds in a single experiment. However, its reliance on affinity-based selection creates a well-known bottleneck: Binding does not guarantee biological function. This limitation is particularly acute for modalities such as targeted protein degradation (TPD), molecular glues, and other mechanisms where cellular context, kinetics, and downstream signaling matter more than binding affinity alone [1]. As a result, many DEL-derived hits require extensive downstream validation, with significant cost and attrition during cell-based follow-up.
Introducing PhenoDEL: Bringing Function into DNA Encoded Library Screening
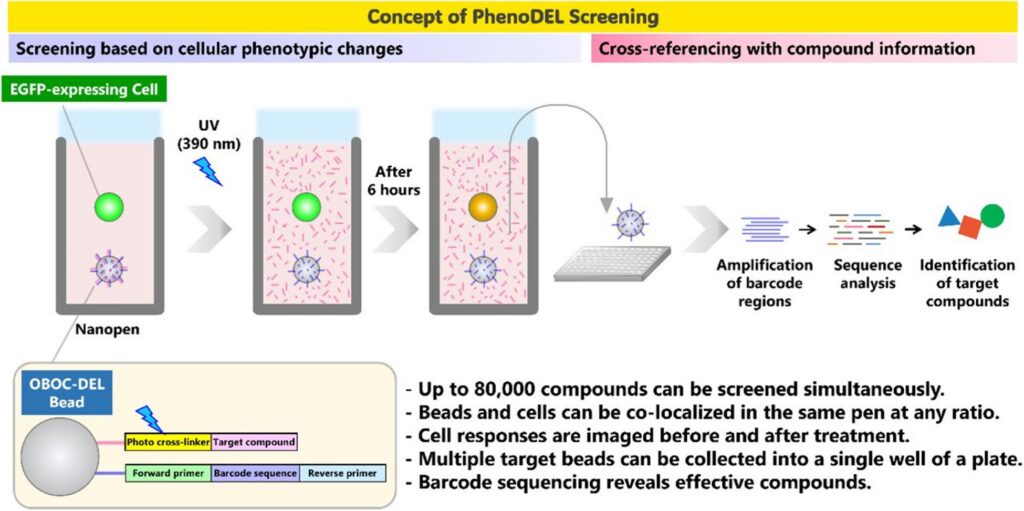
The PhenoDEL workflow addresses this challenge by rethinking how DEL members are screened. Instead of screening DEL compounds as pooled mixtures, the authors employ a solid-phase, OBOCDEL format, where each bead presents a single compound along with a unique DNA barcode. These beads are then:
- Isolated into nanoliter-scale chambers (NanoPen® chambers)
- Colocalized with living reporter cells
- Monitored over time for phenotypic responses (hours to days)
- Decoded by sequencing to identify the active compound
This entire workflow is enabled by the Beacon optofluidic system, which provides deterministic control over bead and cell placement, continuous livecell imaging, and the ability to run sequential functional assays on the same cells [1, 2]. In the study, the authors demonstrate intracellular protein degradation as the phenotypic readout, using a fluorescent reporter system to directly observe compound-induced degradation events at the single-cell level [1].
How Optofluidics Enable Functional DEL Screening
What makes this approach particularly powerful is not only the chemistry, but the optofluidic control that the Beacon platform brings to the problem. Unlike droplet-based or plate-based microfluidic approaches, the Beacon platform enables:
- Precise bead and cell colocalization
- Isolation of individual reactions without crosstalk
- Temporal measurements across hours or days, especially with continuous perfusion
- Multiparameter functional assays on the same cell
- Recovery of cells and/or beads of interest for downstream analysis
These capabilities are foundational to function-first screening, where the goal is not simply to detect a signal, but to understand how and when a compound exerts its effect [2, 3]. In the context of DEL, this means phenotypes can be observed before compound identity is revealed, flipping the traditional screening paradigm while reducing the downstream cost and attrition rates of cell-based assays.
The Beacon® System as a PhenoDEL Screening Engine
While the Beacon system is widely recognized for its impact in antibody discovery, cell line development, and cell therapy workflows, PhenoDEL illustrates something broader: The Beacon system is a general-purpose function-first screening platform. By enabling deterministic control of cells, beads, and reagents at single-cell resolution, the Beacon system makes it possible to:
- Observe biology as it unfolds in real time
- Link function directly to molecular identity
- Run complex, multistep assays without losing context
For small-molecule discovery teams seeking to move beyond binding and into mechanism-driven screening, this represents a meaningful shift.
What PhenoDEL Means for the Future of Small Molecule Screening
The PhenoDEL study is an early demonstration, but its implications are far-reaching. As solid-phase DEL chemistries continue to mature and phenotypic screening becomes increasingly central to drug discovery, platforms that unify throughput, control, and functional depth will be critical.
For HTS and DEL leaders alike, the message is clear:
The future of screening is not just faster – it is more functional.
Optofluidic systems like the Beacon platform are poised to play a central role in that transformation.
References
-
- [1] Onda Y. et al. PhenoDEL as a Novel Screening Strategy Based on Intracellular Protein Degradation Activity. bioRxiv (2025). https://doi.org/10.1101/2025.11.26.690606.
- [2] Bruker Cellular Analysis. Beacon® Optofluidic System Overview. https://brukercellularanalysis.com.
- [3] Bruker Cellular Analysis. Live Single-Cell Functional Analysis with Beacon. Product datasheet and blog resources (2024–2025). https://brukercellularanalysis.com/resources/.