The Race to Select the Best Clones
In the complex world of biologics manufacturing, cell line development (CLD) plays a crucial role in bringing new biologic therapies to patients. A recent survey among industry experts, highlighted in Biotechnology Progress, provides a comprehensive look at current cell line development timelines, techniques, and tools. The article titled “When Will We Have a Clone? An industry perspective on the typical cell line development timeline” explores the methods and challenges inherent to selecting research cell banks (RCB) and lead clones for clinical use. Variations in approach and technology underscore the diversity within the field, though the Beacon® platform is emerging as a prominent industry tool: “According to the survey results… 38% employ the Beacon (Berkeley Lights; Emeryville, CA) [for single-cell cloning of antibody cell lines in industrial CLD laboratories].”
Bruker Cellular Analysis (BCA) has been advancing the Beacon platform’s capabilities since its introduction in 2017, leveraging its world-class assay capabilities, seamless integration, powerful automation, and robust monoclonality assurance to enable confident IND submissions to regulators. This blog delves into how the Beacon platform is reshaping CLD, enabling faster, more reliable clone selection for clinical development.
Reducing Risk Early In the Cell Line Development Process
A central challenge in CLD is balancing the urgency of advancing molecules to clinical stages with the risks of selecting suboptimal clones. The timeline for selecting high-performing clones varies widely based on the methods and tools employed. Much of this variability appears to result from differences in how teams assess and select cell lines from candidate pools. Remarkably, several important assessments of clone performance, such as determining quality and stability, are delayed until months into the process, impacting overall drug development speed (Figure 1).
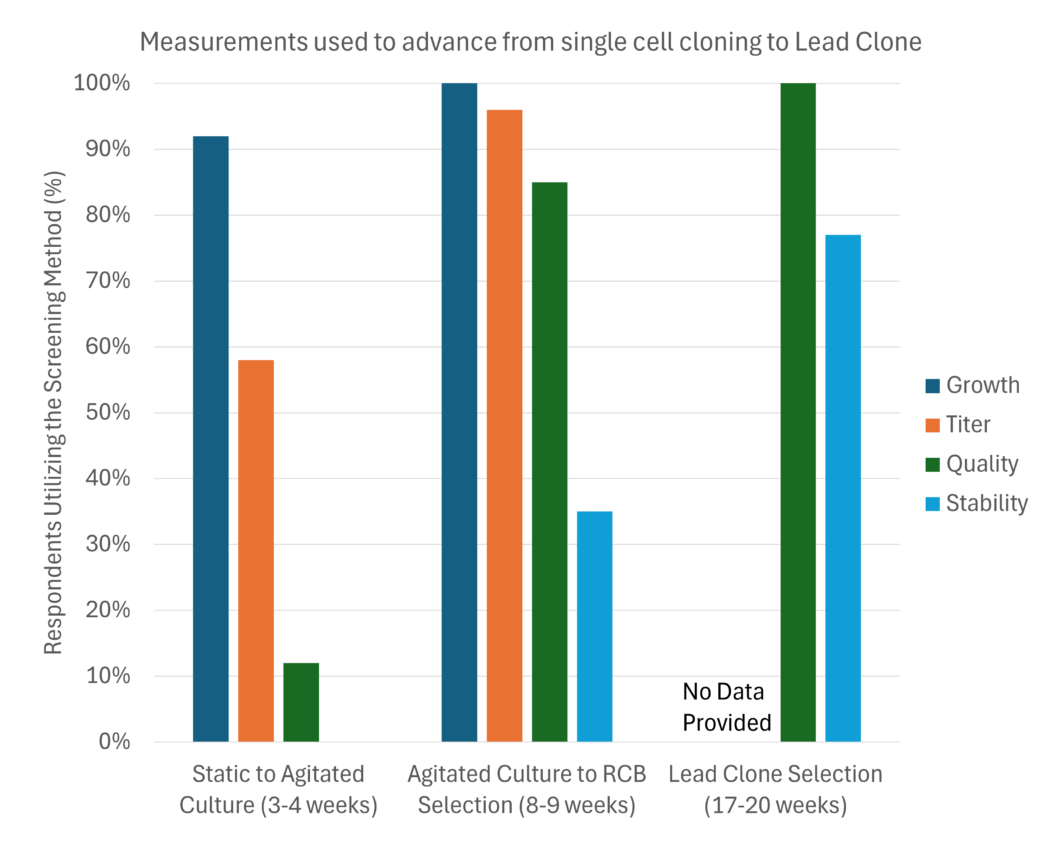
The Beacon CLD platform introduces several advancements to mitigate these risks by offering data-driven, early-stage clone selection. Unlike traditional methods that defer quality and stability assessments, the Beacon platform allows teams to conduct these critical measurements within days to the first month of single-cell cloning, utilizing a robust suite of products and capabilities specifically designed for the CLD community (Table I). This enables early identification of high-quality, stable clones, significantly reducing the risk of late-stage failures.
| Characterization Method | Single Cell Cloning (Day 0) | Week 1 | Static to Agitated Culture (Weeks 4-6) |
|---|---|---|---|
| Monoclonality | Cell Analysis Suite (AI-Enhanced Cell Detection) | Image Analyzer & Assay Analyzer | Image Analyzer & Assay Analyzer |
| Growth | Selective Cell Cloning (Viability) | Image Analyzer & Assay Analyzer | Image Analyzer & Assay Analyzer |
| Titer | Selective Cell Cloning (Cold Capture) | Assay Analyzer & SpotLight™ reagents | Assay Analyzer & SpotLight™ reagents |
| Quality | OptoAssure Assays | OptoAssure Assays | |
| Stability | Population Dynamics |
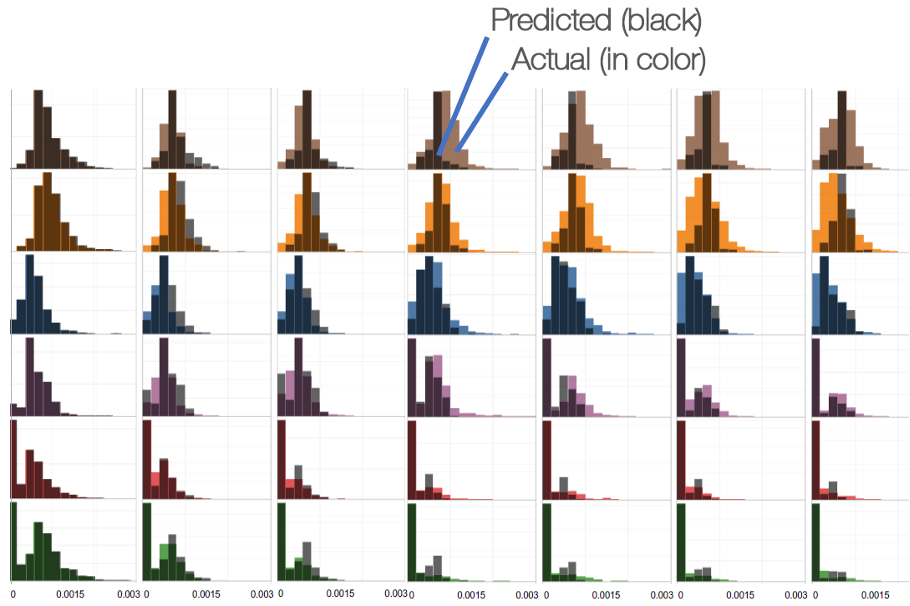
For example, the Beacon platform’s Population Dynamics feature, supported by BCA’s Assay Analyzer software, models clonal behavior based on intraclonal phenotypic heterogeneity revealed through Beacon system’s combination of high-throughput and precise assay capabilities, predicting stability as soon as four weeks into the scale-up process (Figure 2). This capability ensures that teams can make informed decisions early, based not only on productivity and quality information, but also on stability predictions from the earliest stages of the CLD campaign.
Accelerating Biologics Development
Traditional clone selection methods typically face a critical tradeoff between throughput and assay quality. With the Beacon platform’s innovative use of Optofluidics and automated screening in bioreactor-like NanoPen chambers, CLD teams can now analyze thousands of clones within a single workflow using predictive productivity and quality assays. This approach reduces cell line development timelines from several months to weeks, allowing teams to rapidly screen, analyze, and select the most promising clones for further development. Furthermore, the Beacon system has been independently validated by customers, achieving over 99% monoclonality assurance, which provides CLD teams with the confidence needed to advance clones to regulatory submissions.
Returning to the central question of the article, “When will we have a clone?”, the authors address it directly: “The variability in overall timing has more to do with methods used to assess and choose the best cell lines from the many candidates, the risks involved in making decisions with limited data, and how and by whom those decisions are made…” The Beacon CLD platform represents a breakthrough for CLD teams in navigating these challenges, enabling informed, data-driven decisions early in the clone selection process. By allowing CLD teams to assess quality and stability with speed and precision, the Beacon platform empowers users towards regulatory submission and clinical trials as fast as six weeks from single-cell cloning.
As CLD continues to evolve, Bruker Cellular Analysis remains committed to innovating and refining tools that support faster, safer, and more effective biologics development. With robust data insights, high performance, and exceptional speed, the Beacon platform supports CLD teams in overcoming key obstacles, bringing groundbreaking therapies closer to the patients who need them.
