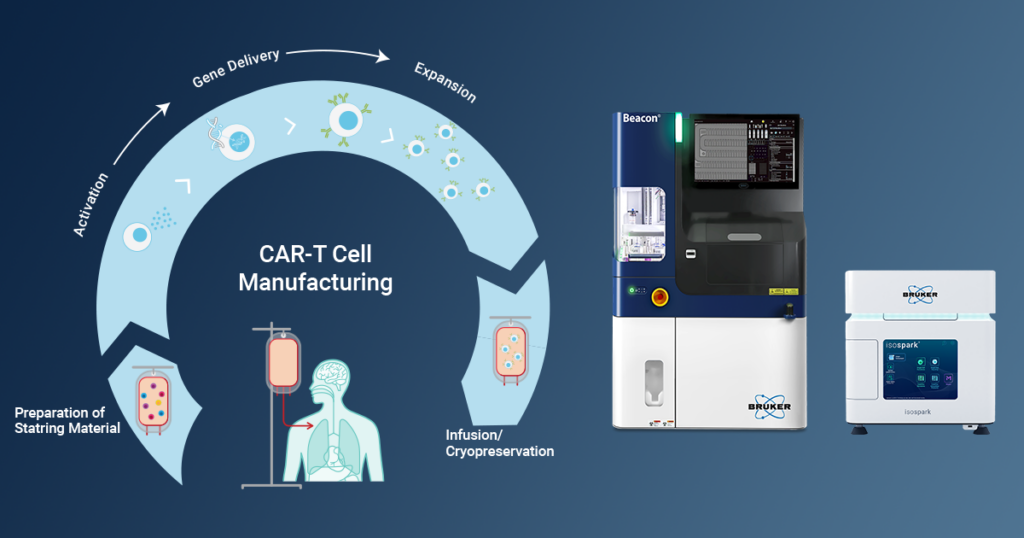
In the rapidly evolving landscape of cancer treatment, chimeric antigen receptor (CAR) T cell therapy has emerged as a groundbreaking immunotherapeutic approach, garnering FDA approval in 2017. However, the journey from initial success to widespread efficacy has been marked by challenges, prompting researchers to continuously reevaluate and refine CAR-T manufacturing processes. A recent review published in the journal Frontiers in Molecular Medicine by researchers at Medical College of Wisconsin sheds light on innovative platforms, including Bruker’s IsoSpark™ and Beacon® instruments, that hold the potential to overcome existing hurdles.
The Evolution of CAR-T Cell Design
In CAR-T therapy development, recent focus has been placed on enhancing CAR constructs and optimizing manufacturing processes to improve in vivo persistence and clinical outcomes. Despite significant advancements, challenges persist in achieving long-term tumor-free survival, reducing therapy-associated malignancies and toxicities, and expanding the range of treatable cancers. The recent review delves into the evolving landscape of CAR-T cell manufacturing, emphasizing the need for precise control over various steps to generate a superior and more efficacious cellular product. Bruker’s IsoSpark and Beacon platforms represent cutting-edge technologies that offer transformative solutions to address these ongoing challenges. These platforms aim to optimize specific steps in the manufacturing process, leading to the development of a more robust and potent CAR-T cell product.
The IsoSpark: Illuminating the Secretory Profile of CAR-T Cells
Bruker’s IsoSpark platform emerges as a game-changer in assessing CAR-T cell functionality. The IsoSpark enables the interrogation of the secretory profile of CAR-T cells at a single-cell resolution, providing a granular view of individual cell function. This platform employs the Polyfunctional Strength Index (PSI) score to quantify the percentage of polyfunctional single cells in a sample, offering standardized metrics that could become an integral part of release criteria for CAR-T cells. This level of detail promises to improve the prediction of clinical response and minimize observed toxicities, ultimately leading to safer and more effective CAR-T cell therapies.
The Beacon: Shedding Light on Single-Cell Functional Assays
Bruker Cellular Analysis’ Beacon platform takes center stage in facilitating single-cell functional assays, addressing the one-size-fits-all approach in current CAR-T cell manufacturing. The Beacon system utilizes non-destructive light to sort cells into NanoPen® chambers, providing the capability to perform both cytokine secretion and cytotoxic assays at the single-cell level. This innovative technology allows researchers to identify cells with the best response to cancer, paving the way for a more personalized and effective approach to CAR-T cell therapy.
The Future of CAR-T Cell Therapy
The evolving field of precision medicine necessitates robust cellular characterizations to uncover manufacturing methods that result in persistent and efficacious CAR-T therapies. Regulatory bodies are also evaluating potential risks, such as the development of T cell malignancies, emphasizing the importance of stringent characterization of CAR-T cell products. Bruker’s Beacon and IsoSpark platforms may hold the key to unlocking the full potential of CAR-T cell therapy by optimizing manufacturing processes. This may lead to more personalized and effective treatment approach.